Pisum
Genetics
2007—Volume
39
Research
Papers
Table 1. Results of allelism test-crosses between the lines
examined.
|
|
Shtambovy |
Rosacrone |
Lupinoid |
JI 5 |
JI 2671 |
JI 2771 |
P 64 |
||
|
Shtambovy |
|
|
|
|
|
|
|
||
|
Rosacrone |
n/a |
|
|
|
|
|
|
||
|
Lupinoid |
n/a |
a |
|
|
|
|
|
||
|
JI 5 |
- |
a |
- |
|
|
|
|
||
|
JI 2671 |
- |
a |
- |
- |
|
|
|
||
|
JI 2771 |
a |
n/a |
- |
- |
n/a |
|
|
||
|
P 64 |
n/a |
n/a |
- |
- |
- |
- |
|
||
Key: a, allelic; n/a, nonallelic; dash, cross not made or
data absent.
Lines 'Rosacrone',
'Lupinoid', JI 5 ('Mummy
Pea'), and JI 2671 are all
allelic. The fasciation in them
is caused by gene Fa localized
on LG IV, as JI 5 ('Mummy
pea', syn. WL 6) is regarded as
type line for fa (11) identical to
one described in Mendel's work
(2, 5). Line JI 2671 also needs
to be designated as fa instead
of fas.
'Lupinoid', JI 5 ('Mummy
Pea'), and JI 2671 are all
allelic. The fasciation in them
is caused by gene Fa localized
on LG IV, as JI 5 ('Mummy
pea', syn. WL 6) is regarded as
type line for fa (11) identical to
one described in Mendel's work
(2, 5). Line JI 2671 also needs
to be designated as fa instead
of fas.
Line P 64 shows no
allelism
with any of the other mutants,
but rather is homozygous at
gene sym28 as stated in (7).
with any of the other mutants,
but rather is homozygous at
gene sym28 as stated in (7).
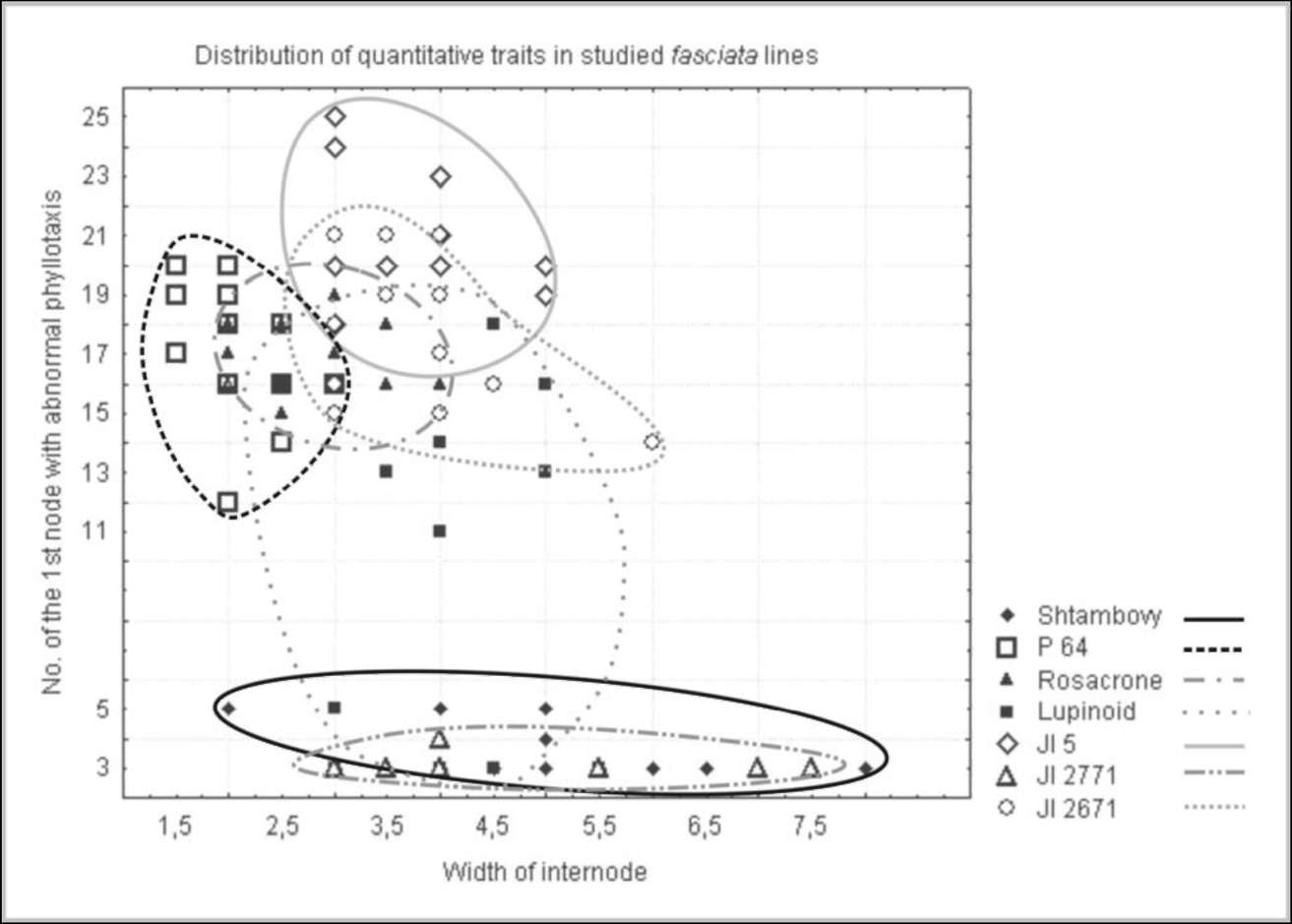
The study of
quantitative
traits in fasciated
lines
provides additional data
confirming the relationship
provides additional data
confirming the relationship
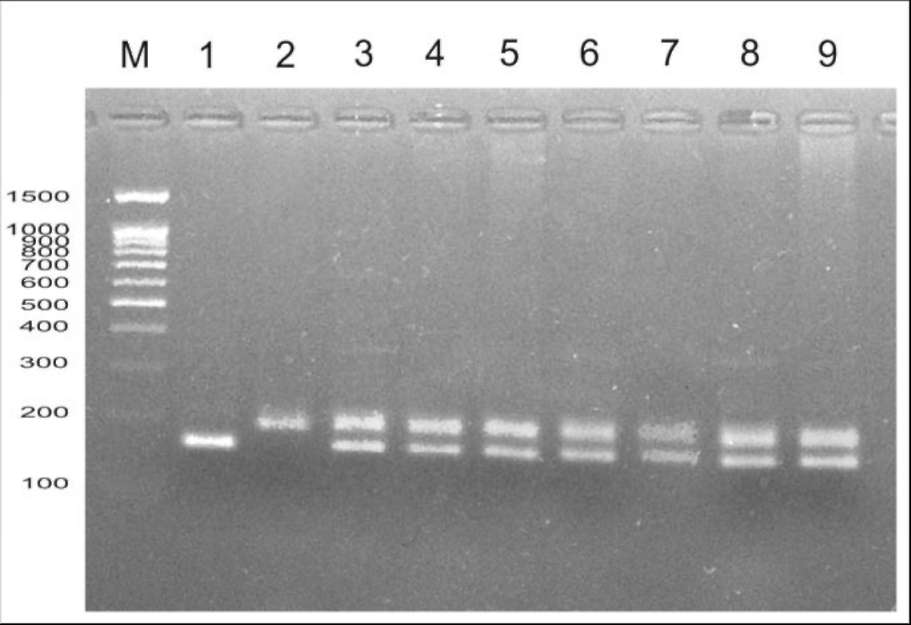
Fig. 1. Confirmation of
hybrid origin of F1 plants from cross between
'Shtambovy' and JI 2771 via amplification of AA122 microsatellite marker.
Key: M, marker of molecular weight (100 bp + 1.5 Kb, Sibenzyme); 1,
'Shtambovy'; 2, JI 2771; 3-9, spectra of individual F hybrids.
'Shtambovy' and JI 2771 via amplification of AA122 microsatellite marker.
Key: M, marker of molecular weight (100 bp + 1.5 Kb, Sibenzyme); 1,
'Shtambovy'; 2, JI 2771; 3-9, spectra of individual F hybrids.
between fasciated lines (Fig.
2).
The two fas forms are
The two fas forms are
characterized with strongly
expressed fasciation resulting in development of widely flattened main
stem and
phyllotaxis distortions, which can be clearly seen even in seedlings. Usually two or three leaves form in the third
node, i.e. true (not scalar cataphylls) leaves exhibit abnormalities in their arrangement. In contrast, fa lines are
weakly fasciated and features of stem flattening and clustering of leaves can be seen only at late stages of
development. The line 'Lupinoid' has unusual leaf arrangement: the formation of leaf whorls is usually observed
on the first nodes and then on 10-11th (and more). Such enhancement of fasciation expression can be explained
by existence of modifying genes altering manifestation of fa in different recombinants. The stem and leaf
arrangement in the P64 line (sym28) are also weakly affected. Such differences were seen even during
observations in the very dry summer of 2007 when all features of fasciation were expressed weaker then usual
due to drought stress.
phyllotaxis distortions, which can be clearly seen even in seedlings. Usually two or three leaves form in the third
node, i.e. true (not scalar cataphylls) leaves exhibit abnormalities in their arrangement. In contrast, fa lines are
weakly fasciated and features of stem flattening and clustering of leaves can be seen only at late stages of
development. The line 'Lupinoid' has unusual leaf arrangement: the formation of leaf whorls is usually observed
on the first nodes and then on 10-11th (and more). Such enhancement of fasciation expression can be explained
by existence of modifying genes altering manifestation of fa in different recombinants. The stem and leaf
arrangement in the P64 line (sym28) are also weakly affected. Such differences were seen even during
observations in the very dry summer of 2007 when all features of fasciation were expressed weaker then usual
due to drought stress.
In conclusion, fasciation for the
lines studied is produced by three independent genes, and its
manifestation
in different genotypes is phenotypically distinguishable. Certain changes in the designation of type lines are
recommended. Further investigations on gene interactions including analysis of F2 and double mutants are
needed to get more information on genetic control of SAM development in pea and higher plants in general.
in different genotypes is phenotypically distinguishable. Certain changes in the designation of type lines are
recommended. Further investigations on gene interactions including analysis of F2 and double mutants are
needed to get more information on genetic control of SAM development in pea and higher plants in general.
17