INTERACTION OF THE det (DETERMINATE) MUTANT WITH
OTHER FLOWERING GENES
Murfet, I.C.
Department of Plant Science, University of
Tasmania
Hobart, Tasmania 7001, Australia
The recessive gene det
(determinate) causes the shoot to terminate in a flower after the
formation of a small number of reproductive nodes (1,2,4,5). Whether the
det mutant is strictly determinate in the botanical sense requires
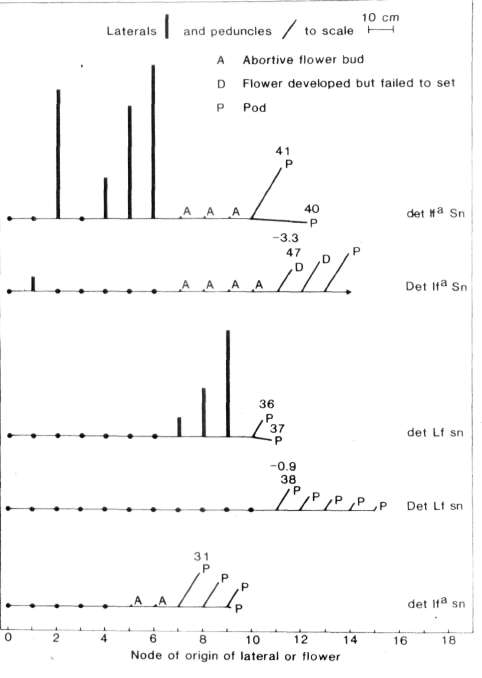
clarification but the fact that the terminal flower is frequently offset
from the vertical (Fig. 1 ) suggests it may have arisen from an axial
flower primordium rather than by direct conversion of the apical meristem
itself.
The present study examined the
interaction between det and several of the other flowering genes in
Pisum. The det mutant was received from Dr Peter Matthews of
the John Innes Institute in the form of line JI 1358 which is tall
(Le) with a late flowering habit indicative of genotype Lf
Sn Dne (see 3). Most JI 1358 plants showed a very large
response to photoperiod indicating the presence of gene Hr but some
displayed only a limited quantitative response to photoperiod indicating
genotype hr. Thus JI 1358 may be heterogeneous for the
Hr-hr gene pair. Line JI 1358 was crossed with Hobart line
69, which is a very early flowering, day neutral dwarf with genotype
lfa E sn Dne hr le,
and 96 F2 plants and several F3 progenies from
lfa or Lf sn
det F2 plants were raised in 9 h short day
conditions (day 23°C, night 16°C).
The results of cross 69 x 1358 gave
no indication that det altered the effect of gene pairs
Lf-lfa, Sn-sn or
Hr-hr on node of flower initiation. For example, in the
F2 node of flower initiation ranged from 6-48 for Det
segregates and 7-52 for det segregates. However, the results of
this small study are not such that they would expose with certainty small
quantitative effects of det on node of flower initiation.
Nevertheless, the results did provide a very clear answer on several
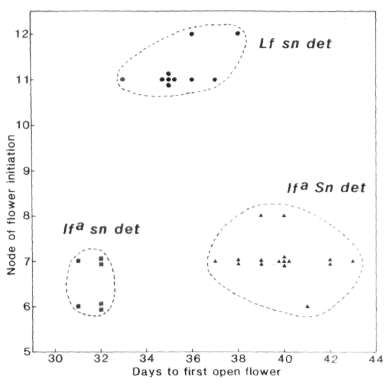
points. For example, segregation for the Lf-lfa gene
pair was clearly apparent in both sn det (Fig. 2) and
Sn det plants on the basis of node of flower initiation.
Likewise segregation of the Sn-sn gene pair was entirely clear on
an lfa det background on the basis of time to first open
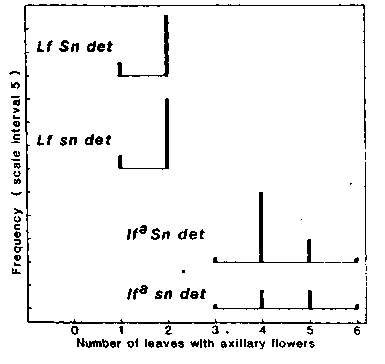
flower (Fig. 2) and several other traits such as flower bud abortion,
peduncle length and branching pattern. For example, lfa sn
det segregates produced few if any lateral branches while
lfa Sn det segregates produced a massive outgrowth of
laterals (Fig. 1) and peduncle length was much longer in the Sn
plants (Fig. 1 ).
Termination of mainshoot growth in
det plants produced several marked effects. With an
lfa Sn background det plants showed a more
precocious and profuse outgrowth of lateral branches than Det
plants (Fig. 1). In lfa Sn det plants these lateral
branches arose from both basal nodes (e.g. nodes 1 and 2) as well as
aerial nodes further up the stem. In Lf sn plants gene
let likewise resulted in the outgrowth of lateral branches but in
this case the outgrowth occurred only from aerial nodes, particularly
those just below the first flower (Fig. 1). In contrast, Lf sn
Det plants were wholly devoid of lateral branches (Fig. 1). Finally,
with an lfa Sn background det brought forward the
time of first open flower by, on average, 4 days compared with that of
Det segregates. This effect was significant at the 0.01 level. The
earlier development of the flower buds on the det plants appears to
have resulted from the availability of nutrients which in Det segregates
were diverted toward the growth of new vegetative organs. Indeed, the
forced growth in lfa Sn det plants meant
the terminal flower in