Pisum Genetics
2010-Volume 42
Research Papers
An archaeobotanical and molecular fairy tale about the early Iron Age Balkan princess and the charred pea
Medovic, A.1, Jovanovic, Z.2, 1Museum of Vojvodina, Novi Sad, Serbia
Stanisavljevic, N.2, 2Univ. of Belgrade, Inst. of Mol. Genet. & Genet. Eng., Belgrade, Serbia
Radovic, S.3, Mikic, A.4, 3University of Belgrade, Faculty of Biology, Belgrade, Serbia
Dordevic, V.4 and Cupina, B.5 4Institute of Field and Vegetable Crops, Novi Sad, Serbia
5University of Novi Sad, Faculty of Agriculture, Novi Sad, Serbia
Pea (Pisum sativum L.) is one of the most important crops in Serbia and other Balkan countries. It is used for human consumption, animal feeding and for various non-food uses such as green manure (1). Apart from the cultivated pea (P. sativum susbsp. sativum), the flora of the southeast regions of Serbia is rich in 'tall pea' (Pisumsativum subsp. elatius (Steven ex M. Bieb.) Asch. & Graebn.), most likely being one of the northernmost borders of its distribution in southeast Europe (2).
Pea is one of the most ancient and the most persistent grain legume crops in the archaeological findings of the Balkans, where the 'agricultural revolution' of Europe began about 9000 years ago. In Serbia, especially in its northern parts, pea is found in tells belonging to Neolithic times, such as the site Gomolava, Bronze Age, with Zidovar and Feudvar, Early Iron Age, with Gomolava, Feudvar and Gradina-upon-Bosut, up to La Tene, represented by the tell of Gomolava (3). Together with lentil (Lens culinaris Medik.), chickpea (Cicer arietinum L.), bitter vetch (Vicia ervilia (L.) Willd.) and some other grain legume and cereal crops, pea travelled up the Danube valley into the continental interior and quickly reached its farthest regions (4).
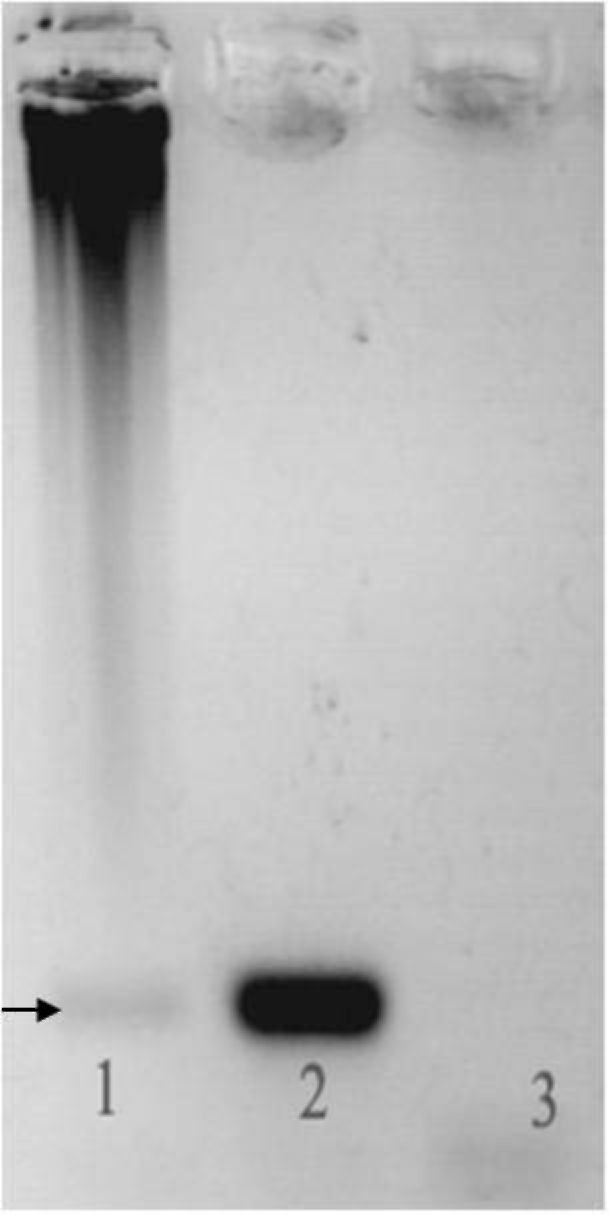
Charred plant remains are possibly the most unexpected type of preserved biological material to have yielded ancient DNA (aDNA), and even though they are fragmented, they still contain information which may help us to understand agricultural or vegetation history (5, 6). Ancient DNA has received much attention since the mid-1980s when the first sequence of an extinct animal species was recovered from a museum specimen (5, 6). Since then, the majority of ancient DNA studies have focused predominantly on animal species, but the investigation of plant aDNA was limited, with the exception of cultivated species found in archaeological sites. There have been studies on charred archaeological samples of other species such as maize and wheat (7). However, there are still problems with charred plant ancient DNA -primarily the enhancement of DNA degradation upon charring. Despite these problems, investigation of ancient plant DNA may provide opportunities for palaeogenetic studies in plants for phylogenetic analysis and species identification (8).
Materials and Methods
The fortified hill settlement of Hissar in Leskovac is a multilevel settlement belonging to the Brnjica cultural group in the Morava Valley and dates back to 1350-1000 B.C. in the Iron Age I (9). At an altitude of 341 masl the Hissar hill has a strategic position over the confluence
of Jablanica and Veternica rivers in South Morava and over the greates
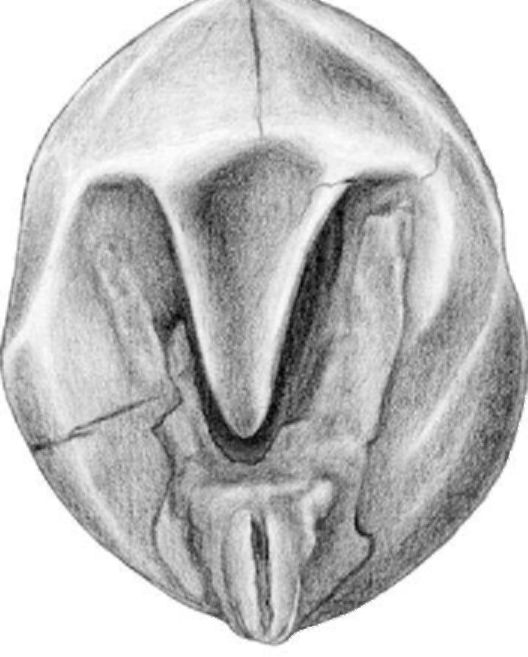
Figure 1. Charred pea seeds from the Iron Age fort Hissar, southern Serbia
part of the Valley of Leskovac. It is fifty kilometers long and forty-five kilometers wide.
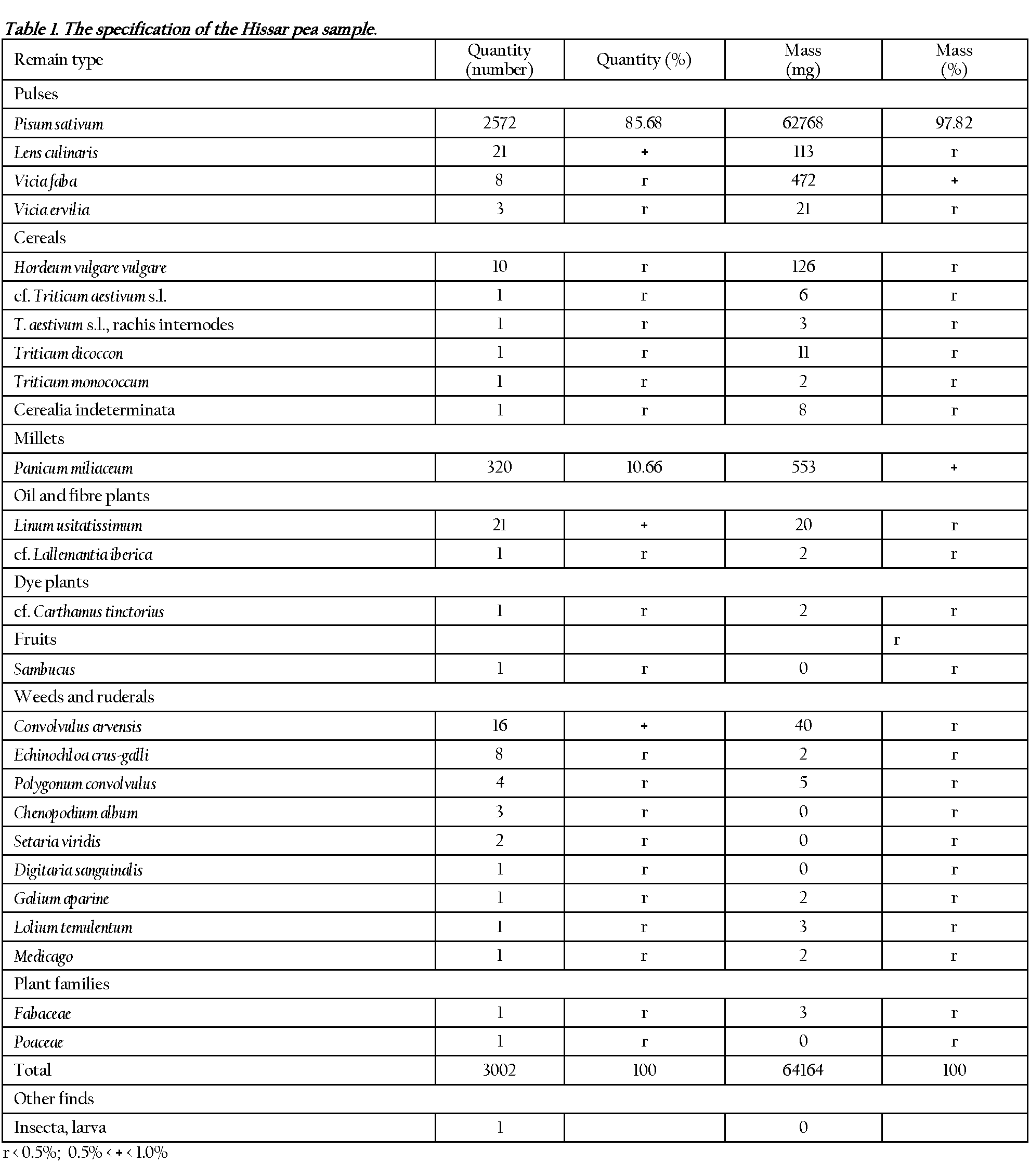
The archaeological excavations at Hissar began in 1999, and in 2005 two rich grain legume samples were collected from the deposits belonging to Brnjica II a level from the 12th century B.C. Flotation of 7 liters of earth substrate yielded 2572 charred seeds of pea (Fig. 1). In one of the previous archaeobotanical reports (10) only one uncertain

35