Pisum
Genetics
2007—Volume
39
Research
Papers
planted. F2 (SGEcrt x
NGB1238)
(Pop1 — 103 individuals) were
grown in quartz sand with full
mineral nutrition added (2) and
analyzed by phenotype (curled root
system) during the 4th week after
planting. F2 (SGEapm x RT9)
(Pop2 — 94 individulas) were grown
in vermiculite with full mineral
nutrition, and formation of stipules
was analyzed on the 7th-10th day
after planting. F2 (SGEFix-- 7 x 87-
18 I-r) (Pop3 — 86 individuals) were
also grown in quartz sand, but with
mineral nutrition lacking NH4NO3
as a source of nitrogen, under
(Pop1 — 103 individuals) were
grown in quartz sand with full
mineral nutrition added (2) and
analyzed by phenotype (curled root
system) during the 4th week after
planting. F2 (SGEapm x RT9)
(Pop2 — 94 individulas) were grown
in vermiculite with full mineral
nutrition, and formation of stipules
was analyzed on the 7th-10th day
after planting. F2 (SGEFix-- 7 x 87-
18 I-r) (Pop3 — 86 individuals) were
also grown in quartz sand, but with
mineral nutrition lacking NH4NO3
as a source of nitrogen, under
inoculation with
Rhizobium
leguminosarum bv. viciae CIAM1026
(23) immediately after planting.
Four week old plants were scored
leguminosarum bv. viciae CIAM1026
(23) immediately after planting.
Four week old plants were scored
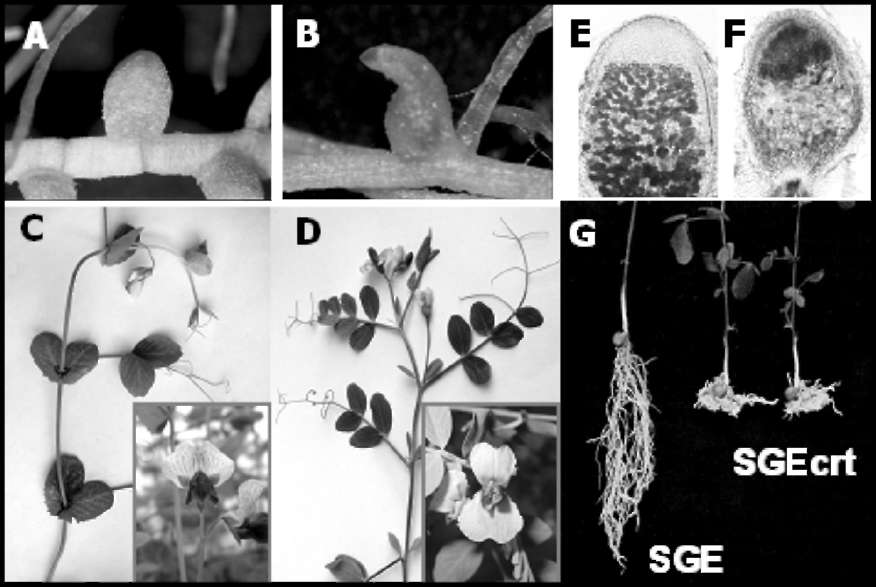
Fig. 1. Phenotypes of crt,
coch and sym27 mutants obtained on line SGE.
A - nodule of SGE; B - abnormal nodule of SGEapm (coch); C - shoot and
flower of SGE; D - shoot and flower of SGEapm (coch): reduced stipules
and deformed flower; E - section of nodule of SGE; F - section of early
senescent nodule of SGEFix--7 (sym27); G - root system of SGE and SGEcrt
(crt) grown in quartz sand (25).
A - nodule of SGE; B - abnormal nodule of SGEapm (coch); C - shoot and
flower of SGE; D - shoot and flower of SGEapm (coch): reduced stipules
and deformed flower; E - section of nodule of SGE; F - section of early
senescent nodule of SGEFix--7 (sym27); G - root system of SGE and SGEcrt
(crt) grown in quartz sand (25).
for the presence or absence
of
functional nodules by observing
functional nodules by observing
their size and color. Because of
difficulties in phenotype determination in the F2,
the analysis was repeated in
the F3, which also provided information on F2 heterozygotes at the sym27 locus.
the F3, which also provided information on F2 heterozygotes at the sym27 locus.
DNA was extracted from leaves of
F2 plants, as well as of parental lines, by a standard CTAB
method (21)
with slight modifications. PCR amplification of DNA markers was performed in thermocyclers Personal Cycler
(Biometra, Germany) and iCycler™ (Bio-Rad, USA). Direct sequencing of PCR products was performed in an
automatic sequencer CEQ™ 8000 Genetic Analysis System (Beckman Coulter, USA). Detected SNPs were
examined for change in recognition sites of endonucleases with use of web-based program dCAPS Finder 2.0.
(20, helix.wustl.edu/dcaps/dcaps.html). Endonucleases for CAPS analysis were supplied by Fermentas
(Lithuania) and SibEnzyme (Novosibirsk, Russia). Fractionation of restriction fragments was performed on
agarose gels (1 — 3%, depending on size of the fragments). Genes for creating EST-markers were chosen by their
location on linkage group V, according to Weeden et al. (30), Brauner et al. (4) and data collected on
http://www.comparative-legumes.org/ (Table 1). Primers had been designed with help from the web-based program
Oligonucleotide Properties Calculator (12, www.basic.northwestern.edu/biotools/oligocalc.html) and
synthesized by Syntol (Moscow, Russia) and Evrogen (Moscow, Russia). Positions of corresponding genes in M.
truncatula had been detected by CViT-BLAST search on http://www.medicago.org/genome/cvit_blast.php (default
parameters, BLASTN and/or BLASTX), and the presence of homologous gene sequence had been confirmed by
pairwise alignment on NCBI BLAST server (http://www.ncbi.nlm.nih.gov/blast/bl2seq/wblast2.cgi) (24).
with slight modifications. PCR amplification of DNA markers was performed in thermocyclers Personal Cycler
(Biometra, Germany) and iCycler™ (Bio-Rad, USA). Direct sequencing of PCR products was performed in an
automatic sequencer CEQ™ 8000 Genetic Analysis System (Beckman Coulter, USA). Detected SNPs were
examined for change in recognition sites of endonucleases with use of web-based program dCAPS Finder 2.0.
(20, helix.wustl.edu/dcaps/dcaps.html). Endonucleases for CAPS analysis were supplied by Fermentas
(Lithuania) and SibEnzyme (Novosibirsk, Russia). Fractionation of restriction fragments was performed on
agarose gels (1 — 3%, depending on size of the fragments). Genes for creating EST-markers were chosen by their
location on linkage group V, according to Weeden et al. (30), Brauner et al. (4) and data collected on
http://www.comparative-legumes.org/ (Table 1). Primers had been designed with help from the web-based program
Oligonucleotide Properties Calculator (12, www.basic.northwestern.edu/biotools/oligocalc.html) and
synthesized by Syntol (Moscow, Russia) and Evrogen (Moscow, Russia). Positions of corresponding genes in M.
truncatula had been detected by CViT-BLAST search on http://www.medicago.org/genome/cvit_blast.php (default
parameters, BLASTN and/or BLASTX), and the presence of homologous gene sequence had been confirmed by
pairwise alignment on NCBI BLAST server (http://www.ncbi.nlm.nih.gov/blast/bl2seq/wblast2.cgi) (24).
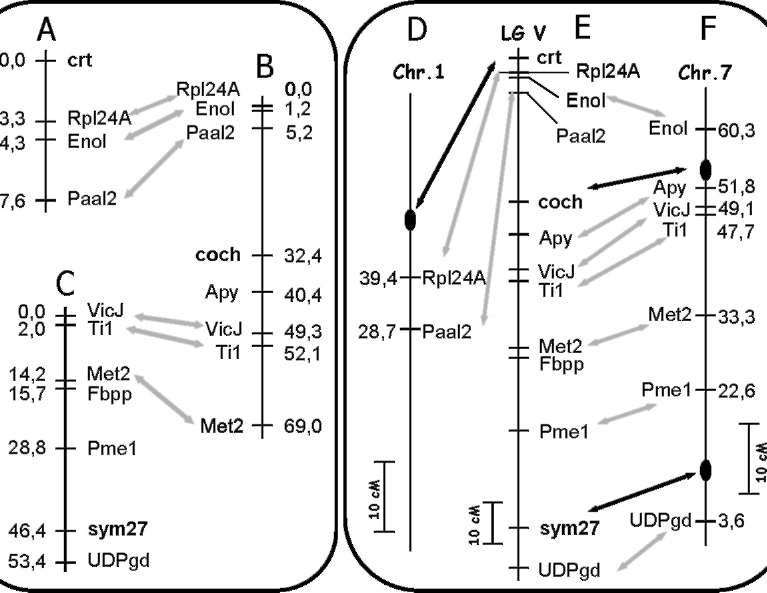
Genetic maps for each cross were
constructed using the program MapL98 (Prof. Yasuo Ukai,
Biometrics
Laboratory, Graduate School of Agricultural Life Science, the University of Tokyo), (default parameters,
LOD > 3.00). Genetic distances between markers were determined by converting the frequency of
recombination events into Kosambi units (15).
Laboratory, Graduate School of Agricultural Life Science, the University of Tokyo), (default parameters,
LOD > 3.00). Genetic distances between markers were determined by converting the frequency of
recombination events into Kosambi units (15).
Results
Genes of interest had been
previously localized on linkage group V (LG V) of pea: crt in
relation to
morphological markers r and tl and the protein marker Sca (26), cochleata in relation to gp and tl (19, 31, cited by
Rozov et al. (22)), and later in relation to the protein markers His1 and Sca (22), and sym27 in relation to
morphological markers gp and Ust (25). Therefore, we have chosen several genes of known position in LG V for
morphological markers r and tl and the protein marker Sca (26), cochleata in relation to gp and tl (19, 31, cited by
Rozov et al. (22)), and later in relation to the protein markers His1 and Sca (22), and sym27 in relation to
morphological markers gp and Ust (25). Therefore, we have chosen several genes of known position in LG V for
20