Pisum
Genetics
2007—Volume
39
Research
Papers
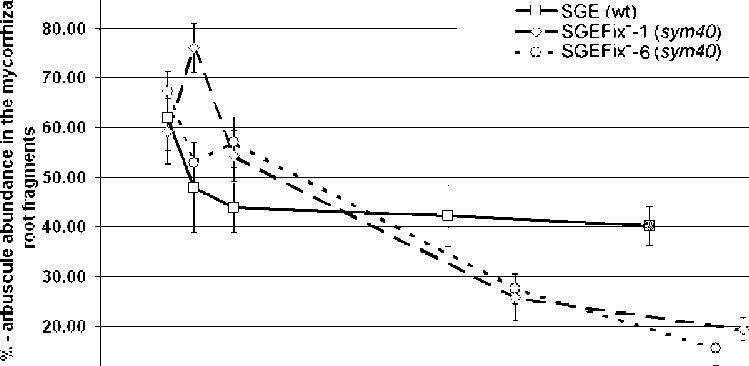
dynamics of
arbuscule
development and turnover was
similar to that described before in
development and turnover was
similar to that described before in
Jacobi et al. (2) (Fig. 2, 3).
Mutant SGEFix:-1. Many
more
appressoria were formed on the
roots of the mutant line SGEFix-- 1
(sym40) than on those of SGE or
appressoria were formed on the
roots of the mutant line SGEFix-- 1
(sym40) than on those of SGE or
SGEFix-- 6 (sym40) (Fig. 1).
Plants
of the mutant line
SGEFix-1
showed an intensity of
colonization close to that of the
wild type at the early time points
(Fig. 2) and high speed of
arbuscule development
accompanied with their fast
turnover (Fig. 3) as was described
previously (2).
showed an intensity of
colonization close to that of the
wild type at the early time points
(Fig. 2) and high speed of
arbuscule development
accompanied with their fast
turnover (Fig. 3) as was described
previously (2).
Mutant SGEFixc-6. In
contrast to
SGE, by eight days of growth in
SGE, by eight days of growth in
NPIS the mutant line SGEFix-- 6
(sym40), exhibited a
similar high
number of appressoria as SGEFix--
1 (sjm40) (Fig. 1). Plants of
SGEFix-- 6 ( sym40) differed from
the other two lines by the reduced
speed of mycorrhization (Fig. 2).
Although the speed of arbuscule
development in the roots of line
SGEFix-- 6 (sym40) is slightly
slower in comparison with the
other mutant line, the process of
arbuscule turnover in this line was
similar to that of the line SGEFix--
1 (sym40) (Fig. 3).
number of appressoria as SGEFix--
1 (sjm40) (Fig. 1). Plants of
SGEFix-- 6 ( sym40) differed from
the other two lines by the reduced
speed of mycorrhization (Fig. 2).
Although the speed of arbuscule
development in the roots of line
SGEFix-- 6 (sym40) is slightly
slower in comparison with the
other mutant line, the process of
arbuscule turnover in this line was
similar to that of the line SGEFix--
1 (sym40) (Fig. 3).
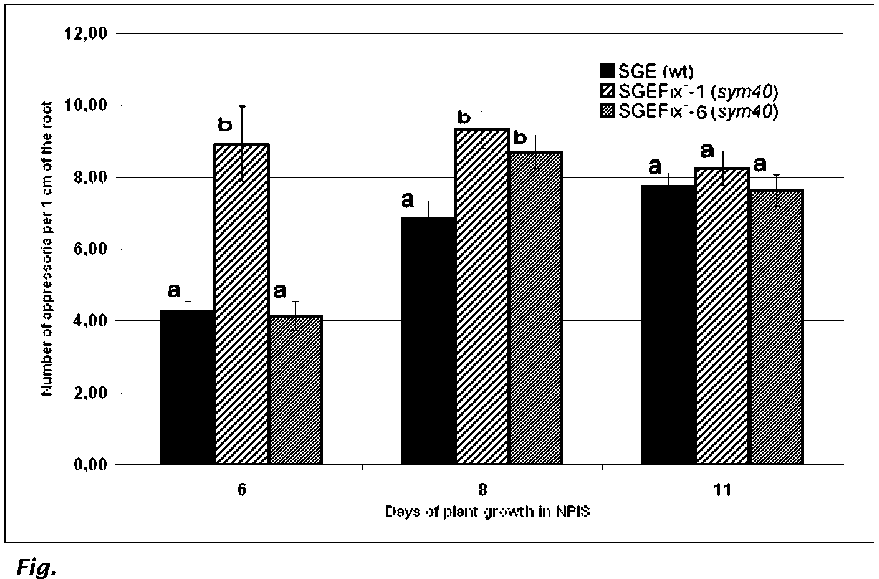
1. Number of appressoria per
1 cm of the root in pea symbiotic
mutants SGEFix-1 (sym40), SGEFix-6 (sym40) and the wild-type line SGE.
Standard errors show variance of mean values at certain time points.
Values marked with the same letters do not statistically significant differ
at PZ0.95 at certain time points.
mutants SGEFix-1 (sym40), SGEFix-6 (sym40) and the wild-type line SGE.
Standard errors show variance of mean values at certain time points.
Values marked with the same letters do not statistically significant differ
at PZ0.95 at certain time points.
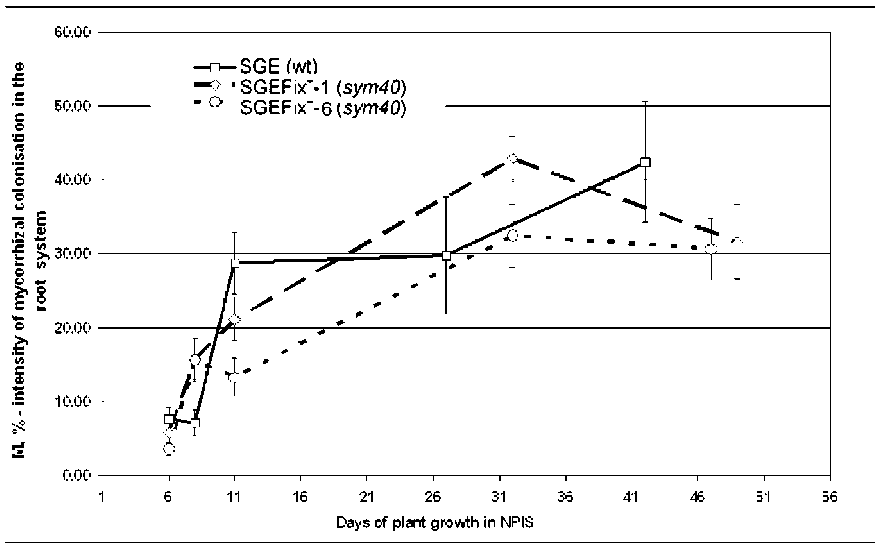
Fig. 2. Intensity of
mycorrhizal colonization in the root system (M%) in
pea symbiotic mutants SGEFix-1 (sym40), SGEFix-6 (sym40) and the wild-
type line SGE. Standard errors show variance of mean values at certain
time points. Values marked with the same color (white, gray) do not
differ at PZ0.95 at certain time points.
pea symbiotic mutants SGEFix-1 (sym40), SGEFix-6 (sym40) and the wild-
type line SGE. Standard errors show variance of mean values at certain
time points. Values marked with the same color (white, gray) do not
differ at PZ0.95 at certain time points.
Discussion
The difference between
the
wild type line SGE and
the
mutant line SGEFix-1 (sym40) in
mutant line SGEFix-1 (sym40) in
number of appressoria in process
of AM development (at six and eight days of plant growth in NPIS)
has
been shown for the first time. In contrast, the mutant line SGEFix-6 (sym40) at six days of growth in
NPIS did not differ significantly from the wild type line SGE, whereas at eight days the two mutant lines
did not differ from each other, but they both differed from the wild type line. Intensity of mycorrhizal
colonization (M%) in the root system of the both mutant lines was similar until eight days of plant growth
in NPIS, but after eight days statistically significant differences between these lines existed. All the lines
differed from each other in dynamics of arbuscule development and turnover, especially at the early time
been shown for the first time. In contrast, the mutant line SGEFix-6 (sym40) at six days of growth in
NPIS did not differ significantly from the wild type line SGE, whereas at eight days the two mutant lines
did not differ from each other, but they both differed from the wild type line. Intensity of mycorrhizal
colonization (M%) in the root system of the both mutant lines was similar until eight days of plant growth
in NPIS, but after eight days statistically significant differences between these lines existed. All the lines
differed from each other in dynamics of arbuscule development and turnover, especially at the early time
14