Pisum Genetics
2006—Volume 38
Research Papers
Two new pea mutations simultaneously affecting tendril and leaflet shape and plant internode length.
S. M. Rozov Inst. of Cytol. and Genet.
Siberian Branch of Russ. Acad. of Sci., Novosibirsk, Russia
During the screening of the EMS-treated pea line SGE, M2 progeny, the two new mutants SGE–0284 and SGE–1003 were isolated, characterized with the effect on tendrils and leaflet shape and also the internode length. These mutants are shown at Fig. 1.

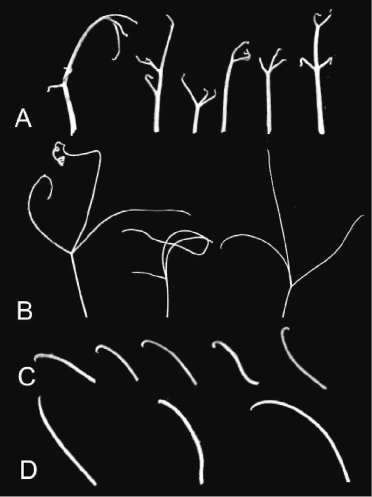
Fig. 1. SGE-0284 and SGE-1003 plants: A – normal SGE Fig 2. Leaves of the SGE-1003 mutant (brtl) —A, B;
plant; B – SGE-0284 mutant; C – SGE-1003 mutant. leaves of the 0284 mutant (htl) —C, D; and the leaves
Note: the internode length of the mutants is slightly of the normal SGE parental plant — E. (SGE-0284) and strictly (SGE-1003) diminished compared to the parental normal line, SGE.
The leaflets of the SGE–0284 and SGE–1003 mutants are also altered in shape: both of them have blunt tips, sometimes with a slight notch at the edge of a tip. Moreover, the SGE–1003 mutant has slightly raised veins on the leaflets, and the leaflets are somewhat insecatus. Fig. 2 presents the view of the mutants leaves.
However, the most striking changes in phenotype of the mutants plants concerned the shape of the tendrils. SGE–0284 possesses the deformed tendril tip—it looks like a hook or a chrochet needle (Fig. 3C), so I propose the symbol htl (hooked tendril) for this mutation. SGE–1003 mutant has an incrassate tendril base with the tendrils being strongly reduced in length. Lateral tendrils are incrassate, strongly hooked, and
17