Variation
for pea seed protein concentration in the USDA Pisum core collection
Coyne,
C.J.1, Grusak, M.A.2, 1USDA-ARS,
WRPIS, Pullman, WA,
USA
Razai,
L.1 and Baik, B.-K.3
2USDA-ARS Children's
Nutrition Res. Center, Houston, TX,
USA
3Dept. of Crop and Soil Sci., Washington State Univ., Pullman,
WA, USA
Introduction
Food legumes are important sources of protein, complex carbohydrates,
vitamins, and minerals in the diets of millions of people (12).
While cereals supply nearly 50% of the protein in the human diet, an
unfavorable balance in amino acids (poor in lysine) requires complementary
protein sources (12). Legumes are
good complements to cereals, as they are rich in lysine, but poor in sulfur
containing amino acids (methionine and cysteine) (12).
Pea seed proteins are composed of albumins and globins which separate
into two major fractions: the 7S vicilin and convicilin fraction, and an 11S
fraction that is predominantly composed of legumin (1).
In this study, we characterized the total seed protein concentration of
480 accessions from the USDA Pisum
core collection. The complete data
set, which is summarized in this article, is available through the internet (http://www.ars-grin.gov/npgs/)
or by contacting the curator (coynec@wsu.edu).
Materials
and Methods
Plant
material
The USDA Pisum core collection
(504 accessions) was used as the source of germplasm (9).
However, it should be noted that many of the accessions in the Pisum
core collection are mixtures of diverse germplasm.
Because we wished to avoid mixed samples for our quantitative analyses,
randomly selected seeds of a single seed phenotype were chosen from each
accession and documented for planting. For
the most part, this resulted in plants with uniform characteristics within each
planted accession. At harvest, if more than one plant phenotype was evident,
seeds were selected from one plant phenotype only (usually the phenotype with
the most plants, or the highest seed yield).
Also, seeds harvested were compared to the original seeds to verify they
were the same phenotype as planted. Phenotypic data were collected on harvested
seeds of each accession, and these characteristics are noted in the GRIN
descriptor dataset listed with the seed protein concentration (Seed Coat Color,
Seed Coat Coloration Pattern, Smooth vs.Wrinkled Seeds, Cotyledon Color) (www.ars-grin.gov/cgi-bin/npgs).
Growth
conditions
Six plants of each accession were grown in 5L black plastic pots filled
with a synthetic soil mix composed of 2 parts Metro-Mix 360 (Scotts-Sierra
Horticultural Products Co., Marysville, Ohio) and 1 part medium grade
vermiculite (Strong-Lite Medium Vermiculite, Sun Gro Horticulture Co, Seneca
Illinois). Plants were grown in a
controlled environment greenhouse with a temperature regime of 22 ± 3°
C/day and 20 ±
3°
C/ night, with a relative humidity ranging from 45% to 65% throughout the
day/night cycle. Sunlight was
supplemented with metal halide lamps, set to a 15-h day, 9-h night cycle (lights
on at 700 h). In order to maintain
an adequate supply of all mineral nutrients, a complete fertilizer mixture was
provided to each pot on a daily basis. Pots were irrigated with an automated
drip irrigation system (one drip line to each pot); the system was regulated
with a timer that delivered nutrient solution twice a day (younger plants) or
three times a day (older plants) in sufficient quantity to saturate the soil
mass at each irrigation. The
nutrient solution contained the following concentrations of mineral salts: 1.0
mM KNO3
,
0.4 mM Ca(NO3)2
,
0.1 mM MgSO4 ,
0.15 mM KH2PO4 and
25
:M CaCl2,
25 :M
H3BO3,
2 :M
MnSO4,
2 :M
ZnSO4,
0.5 :M
CuSO4,
0.5 :M
H2MoO4
,
0.1 :M
NiSO4,
1 :M
Fe(III)-N,
N’-ethylenebis[2-(2-hydroxyphenyl)-glycine]
(Sprint 138; Becker-Underwood, Inc., Ames, Iowa, USA).
We thus attempted to maintain all essential minerals at sufficient,
non-toxic levels in the soil.
Seed Samples
Plants were grown to maturity and all seeds were collected and combined
from the six plants grown for each accession.
Combined seeds were counted, dried to zero moisture in a 70 C oven, and
weighed, in order to calculate 100 seed weights.
Each combined seed sample was ground to a fine powder using a coffee
grinder, prior to nitrogen analyses. Measurements
were conducted on 480 of the 504 accessions in the USDA Pisum
core collection.
Seed nitrogen analyses and protein
calculations
Seed nitrogen concentrations were determined using a LECO FP-528
Nitrogen/Protein Determinator (Leco Corp., St. Joseph, MI, USA), according to
the manufacturer’s instruction manual. Weighed
aliquots of EDTA (ethylenediamine tetraacetic acid) were used as nitrogen
standards to calibrate the instrument. Two
sub-samples (0.15 g each) of each accession were analyzed for nitrogen
concentration; each sample was measured two times internally in the instrument
with the average reported to the operator. The
two sub-sample averages were then averaged to get a nitrogen concentration value
for each accession. No sub-sample
nitrogen values for any accession varied by more than 5%.
Protein concentrations were calculated using a conversion factor of 5.44
([seed nitrogen concentration] x
5.44 = [seed protein concentration]), a multiplier determined by Mossé (6) as
an average value for pea (based on 33 samples).
This multiplier is specific for pea, as it takes into account the actual
amino acid composition of pea seeds, and the nitrogen weight percentage of those
amino acids. Comparison between seed
protein concentration and 100 seed weight was calculated using Pearson’s
correlation (7).
Results
 Once the nitrogen analyzer was calibrated, the readings for the two
samples per accession were very similar. Using
a tolerance level of a maximum 5% difference between samples, no accession
required repeat analysis. The seed
used in this study were grown under controlled conditions, which should
significantly reduce the large effect environment can have on pea seed protein
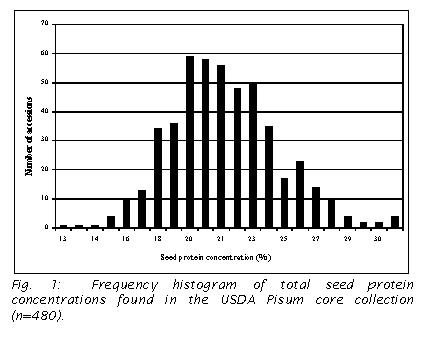
concentration (5). Protein concentration varied over two-fold in the accessions
tested with the highest percentage of 30.93% and lowest of 12.38 % in the
accessions tested. The results are
summarized in a frequency histogram (Fig. 1).
The mean seed protein concentration from round seed (426 accessions) was
20.62 and the mean of the wrinkled seed was higher at 23.76 (51 accessions).
The accessions with the ten highest and ten lowest seed protein
concentrations and their morphological characteristics are presented in Table 1,
many with acceptable agronomic characteristics like white flower and clear seed
coats. Table 2 summarizes the
protein concentrations of the taxons of Pisum
included in this study. While the
sample size between taxon is large (from 1 accession to 437 accessions), the
mean seed protein concentrations are very similar (Table 2).
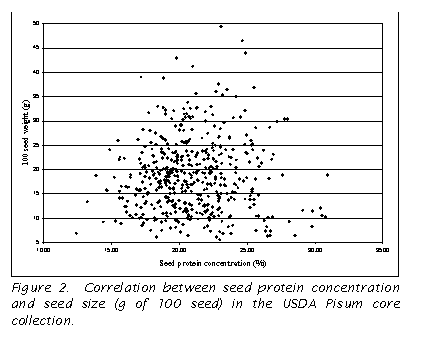
A comparison of the seed size (100 seed weight in g) and seed protein
concentration was conducted, and no correlation was found for the USDA Pisum
core (r=0.01; Figure 2). The
complete data set and accessions are available through the internet (http://www.ars-grin.gov/npgs/)
or by contacting the curator (coynec@wsu.edu).
Once the nitrogen analyzer was calibrated, the readings for the two
samples per accession were very similar. Using
a tolerance level of a maximum 5% difference between samples, no accession
required repeat analysis. The seed
used in this study were grown under controlled conditions, which should
significantly reduce the large effect environment can have on pea seed protein
concentration (5). Protein concentration varied over two-fold in the accessions
tested with the highest percentage of 30.93% and lowest of 12.38 % in the
accessions tested. The results are
summarized in a frequency histogram (Fig. 1).
The mean seed protein concentration from round seed (426 accessions) was
20.62 and the mean of the wrinkled seed was higher at 23.76 (51 accessions).
The accessions with the ten highest and ten lowest seed protein
concentrations and their morphological characteristics are presented in Table 1,
many with acceptable agronomic characteristics like white flower and clear seed
coats. Table 2 summarizes the
protein concentrations of the taxons of Pisum
included in this study. While the
sample size between taxon is large (from 1 accession to 437 accessions), the
mean seed protein concentrations are very similar (Table 2).
A comparison of the seed size (100 seed weight in g) and seed protein
concentration was conducted, and no correlation was found for the USDA Pisum
core (r=0.01; Figure 2). The
complete data set and accessions are available through the internet (http://www.ars-grin.gov/npgs/)
or by contacting the curator (coynec@wsu.edu).
Table 1.
Summary of selected characteristics for those accessions in the USDA Pisum
core collection that exhibited the ten highest and ten lowest seed protein
concentrations.
|
Accession
|
Taxon
|
Seed % Protein
|
Country
|
Flower
|
Cotyledon
|
Node to First Flower
|
Seed Surface
|
|
PI 357292
|
Pisum
sativum
|
30.93
|
Yugoslavia
|
white
|
green
|
9-10
|
wrinkled
|
|
PI 343978
|
Pisum
sativum
subsp.
elatius
|
30.77
|
Turkey
|
pigmented
|
yellow
|
12-15
|
round
|
|
PI 137118
|
Pisum
sativum
|
30.51
|
Canada
|
pigmented
|
yellow
|
13-17
|
round
|
|
PI 288024
|
Pisum
sativum
|
30.4
|
France
|
white
|
green
|
6
|
round
|
|
PI 102887
|
Pisum
sativum
|
29.81
|
China
|
white
|
yellow
|
9-10
|
round
|
|
PI 165949
|
Pisum
sativum
|
29.75
|
India
|
pigmented
|
yellow
|
10-13
|
round
|
|
PI 261671
|
Pisum
sativum
|
29.08
|
Netherlands
|
white
|
yellow
|
10-13
|
round
|
|
PI 125840
|
Pisum
sativum
|
28.51
|
Afghanistan
|
pigmented
|
yellow
|
13-16
|
round
|
|
PI 272207
|
Pisum
sativum
|
28.08
|
Greece
|
pigmented
|
yellow
|
no data
|
round
|
|
PI 103709
|
Pisum
sativum
|
27.95
|
India
|
white
|
green
|
10-11
|
round
|
|
PI 203944
|
Pisum
sativum
|
15.48
|
Mexico
|
white
|
yellow
|
16-19
|
round
|
|
PI 358610
|
Pisum
sativum subsp abyssinicum
|
15.28
|
Ethiopia
|
pigmented
|
yellow
|
10
|
round
|
|
PI 324706
|
Pisum
sativum
|
15.21
|
Romania
|
pigmented
|
yellow
|
17-21
|
round
|
|
PI 204306
|
Pisum
sativum
|
14.88
|
Australia
|
pigmented
|
yellow
|
20-22
|
round
|
|
PI 358623
|
Pisum
sativum
|
14.67
|
Ethiopia
|
pigmented
|
yellow
|
15-18
|
round
|
|
PI 204307
|
Pisum
sativum
|
14.62
|
Australia
|
white
|
yellow
|
11-14
|
round
|
|
PI 134271
|
Pisum
sativum
|
14.37
|
Afghanistan
|
pigmented
|
yellow
|
12-14
|
round
|
|
PI 188698
|
Pisum
sativum
|
13.87
|
Nigeria
|
pigmented
|
green
|
15-20
|
round
|
|
PI 356986
|
Pisum
sativum
|
13.20
|
India
|
pigmented
|
yellow
|
12-13
|
round
|
|
PI 222071
|
Pisum
sativum
|
12.38
|
Afghanistan
|
pigmented
|
yellow
|
12-19
|
round
|
Table 2.
Taxon summary of the pea seed protein concentrations measured in the USDA
Pisum core collection.
|
Taxon
|
Number of
accessions
|
Protein
minimum
|
Protein
maximum
|
Protein mean (s.e.)
|
|
Pisum
sativum
|
437
|
12.38
|
30.93
|
20.93 (0.15)
|
|
Pisum
sativum ssp elatius
|
027
|
17.62
|
30.77
|
22.25 (0.56)
|
|
Pisum
sativum ssp abyssinicum
|
713
|
15.28
|
23.11
|
19.39 (0.71)
|
|
Pisum
sativum subsp arvense
|
002
|
18.17
|
23.59
|
20.88
|
|
Pisum
sativum var pumillio
|
001
|
n.a.
|
n.a.
|
19.34
|
Discussion
Protein concentration in many biological tissues, such as seeds, is often
measured indirectly as percent nitrogen. Protein
concentration is then calculated from the nitrogen value using a
nitrogen-to-protein conversion factor. For
many foods, a factor of 6.25 is generally used, a value based on the average
nitrogen percentage of a mix of common amino acids (11).
However, because different amino acids vary in their nitrogen
percentages, and different proteins contain varying mixtures of amino acids, it
is more accurate to use a conversion factor that is based on the specific
proteins contained in a given food (3, 6, 11).
For this reason, a conversion factor of 5.44 was used in this study for
all protein calculations, a value derived from actual amino acid analyses of
several pea genotypes (6).
The seed protein concentration analysis of the USDA Pisum
core collection revealed values ranging from 30.93% to 12.38% (Table 1).
These values are comparable to the values of 34.1% to 14.5% for Pisum
sativum seed obtained by Savage and Deo (8), especially when their values
are recalculated with a 5.44 conversion factor instead of the 6.25 factor that
was used (their recalculated range would be 29.7% to 12.6%).
Similarly, our values for Pisum
sativum subsp. abyssinicum
accessions overlap with those of Yemane and Skjelvåg (12), who found
concentrations of 19.9% and 20.5% (recalculated with 5.44 conversion factor) for
whole seed of two abyssinicum
cultivars.
 The 2.5-fold range reported here (30.93% to 12.38 %) is in contrast to a
field study conducted with 1071 USDA accessions in 1975 (4), in which only a
1.4-fold variation in seed protein concentration was found (26.9% to 19.7%;
recalculated with 5.44 conversion factor). Studies
of seed protein concentration levels in a number of legume crops have shown
extreme sensitivity to the environment (5). The controlled
conditions of the greenhouse culture versus the field environment may be a
possible explanation for the differences in the two studies using USDA pea
germplasm. A slightly higher,
three-fold variation was found in a field study of 255 accessions held in the
John Innes pea germplasm collection (5). In
that report (5), the protein values (presented as a-amino
nitrogen) were negatively skewed (i.e., more to the lower concentrations; their
Fig. 33.2), unlike the more symmetrical distribution found in the present study
(Fig. 1), and this appears to account for their broader variation in protein
values. Interestingly, the
accessions analyzed in that study also represented a greater diversity of Pisum subspecies. No
accessions, for instance, of Pisum fulvum
or Pisum sativum subsp. transcaucasicum
are included in the current seed protein concentration dataset (Table 2).
The 2.5-fold range reported here (30.93% to 12.38 %) is in contrast to a
field study conducted with 1071 USDA accessions in 1975 (4), in which only a
1.4-fold variation in seed protein concentration was found (26.9% to 19.7%;
recalculated with 5.44 conversion factor). Studies
of seed protein concentration levels in a number of legume crops have shown
extreme sensitivity to the environment (5). The controlled
conditions of the greenhouse culture versus the field environment may be a
possible explanation for the differences in the two studies using USDA pea
germplasm. A slightly higher,
three-fold variation was found in a field study of 255 accessions held in the
John Innes pea germplasm collection (5). In
that report (5), the protein values (presented as a-amino
nitrogen) were negatively skewed (i.e., more to the lower concentrations; their
Fig. 33.2), unlike the more symmetrical distribution found in the present study
(Fig. 1), and this appears to account for their broader variation in protein
values. Interestingly, the
accessions analyzed in that study also represented a greater diversity of Pisum subspecies. No
accessions, for instance, of Pisum fulvum
or Pisum sativum subsp. transcaucasicum
are included in the current seed protein concentration dataset (Table 2).
Jermyn and Slinkard (4) presented field data demonstrating that as
protein increased, yields decreased, when they assessed the USDA pea collection
in the 1970’s. Although yield was
not measured in the current study, no correlation was found between the related
trait, seed size, and seed protein concentration (Fig. 2).
Additionally, a small replicated trial conducted in one location and one
year (2004) of high yielding cultivars now in production in Washington State
indicates most have seed protein concentrations in the higher range (~ 25 to
28%) (Coyne, unpublished). Thus, it
appears that seed protein concentration can be enhanced independently of yield
and/or seed size in pea.
The original USDA Pisum core collection was selected using only geography (country of
origin) and flower color as trait variables (9).
Seed protein concentration was not used in the selection process.
Nonetheless, based on the seed protein concentrations reported in this
investigation (Fig. 1, Tables 1, 2), relative to other studies (4, 5), it would
appear that the core adequately represents seed protein variability found in
pea. Therefore, these protein values
have recently been included with other trait data to reduce the size of the
original core to achieve a desired 10% representation of the entire USDA Pisum collection (2).
Several QTL studies on the heritability of pea seed protein concentration
(9), along with recent studies using Medicago
truncatula to study legume seed proteins (3), are increasing our
understanding of the genetics of this important component of pea seeds.
The accessions in Table 2 may aid in the future discovery of useful
alleles for breeding enhanced seed protein levels in this crop, or could prove
valuable for mapping novel regulatory genes associated with increased seed
protein concentration in pea.
Acknowledgments:
This work was supported by USDA-ARS Projects # 5348-21000-020-00D (Coyne)
and # 6250-21520-042-00D (Grusak), and through USDA funds provided by the Pisum
Crop Germplasm Committee (to Grusak).
1. Casey, R. and Domoney, C. 1985.
In:
Hebblethwaite, P.D., Heath, M.C. and Dawkins, T.C.K.
(eds.)
The Pea Crop. Butterworths,
London
, pp 359-368.
2. Coyne, C.J., Brown, A.F.,
Timmerman-Vaughan, G.M., McPhee, K.E. and Grusak, M.A. 2005.
Pisum Genetics 37: 1-4.
3. Djemel, N., Guedon, D.,
Lechevalier, A., Salon, C., Miquel, M., Prosperi, J.-M., Rochat, C. and Boutin,
J.-P. 2005. Plant
Physiol. Biochem. 43: 557-566.
4. Jermyn, W.A. and Slinkard,
A.E. 1977. Legume
Res. 1: 33-37.
5. Mathews, P. and Arthur, E. 1985.
In: Hebblethwaite, P.D., Heath, M.C.
and Dawkins, T.C.K. (eds.)
The Pea Crop. Butterworths,
London
, pp. 369-381.
6. Mossé, J. 1990.
J. Agric. Food Chem. 38: 18-24.
7. SAS 9.1. 2002-2003.
SAS Institute,
Cary
,
NC
,
USA
.
8. Savage, G.P. and Deo, S.
1989. Nutr. Abstr. Rev.
Ser. A 59: 65-88.
9. Simon, C.J. and Hannan, R.M.
1995. HortSci.
30: 907.
10.
Tar’an, B., Warkentin, T., Somers, D.J., Miranda, D., Vandenberg, A.,
Blade, S. and Bing, D. 2004. Euphytica
136: 297-306.
11.
Tkachuk, R. 1977. In: Hulse,
J.H., Rachie, K.O. and Billingsley, L.W. (eds.) Nutritional
Standards and Methods of Evaluation for Food Legume Breeders. International
Development Research Centre,
Ottawa
,
Canada
, pp. 78-82.
12.
Yemane, A. and Skjelvåg, A.O. 2003.
Plant Foods Human Nutr.
58: 275–283.
 Once the nitrogen analyzer was calibrated, the readings for the two
samples per accession were very similar. Using
a tolerance level of a maximum 5% difference between samples, no accession
required repeat analysis. The seed
used in this study were grown under controlled conditions, which should
significantly reduce the large effect environment can have on pea seed protein
concentration (5). Protein concentration varied over two-fold in the accessions
tested with the highest percentage of 30.93% and lowest of 12.38 % in the
accessions tested. The results are
summarized in a frequency histogram (Fig. 1).
The mean seed protein concentration from round seed (426 accessions) was
20.62 and the mean of the wrinkled seed was higher at 23.76 (51 accessions).
The accessions with the ten highest and ten lowest seed protein
concentrations and their morphological characteristics are presented in Table 1,
many with acceptable agronomic characteristics like white flower and clear seed
coats. Table 2 summarizes the
protein concentrations of the taxons of Pisum
included in this study. While the
sample size between taxon is large (from 1 accession to 437 accessions), the
mean seed protein concentrations are very similar (Table 2).
A comparison of the seed size (100 seed weight in g) and seed protein
concentration was conducted, and no correlation was found for the USDA Pisum
core (r=0.01; Figure 2). The
complete data set and accessions are available through the internet (http://www.ars-grin.gov/npgs/)
or by contacting the curator (coynec@wsu.edu).
Once the nitrogen analyzer was calibrated, the readings for the two
samples per accession were very similar. Using
a tolerance level of a maximum 5% difference between samples, no accession
required repeat analysis. The seed
used in this study were grown under controlled conditions, which should
significantly reduce the large effect environment can have on pea seed protein
concentration (5). Protein concentration varied over two-fold in the accessions
tested with the highest percentage of 30.93% and lowest of 12.38 % in the
accessions tested. The results are
summarized in a frequency histogram (Fig. 1).
The mean seed protein concentration from round seed (426 accessions) was
20.62 and the mean of the wrinkled seed was higher at 23.76 (51 accessions).
The accessions with the ten highest and ten lowest seed protein
concentrations and their morphological characteristics are presented in Table 1,
many with acceptable agronomic characteristics like white flower and clear seed
coats. Table 2 summarizes the
protein concentrations of the taxons of Pisum
included in this study. While the
sample size between taxon is large (from 1 accession to 437 accessions), the
mean seed protein concentrations are very similar (Table 2).
A comparison of the seed size (100 seed weight in g) and seed protein
concentration was conducted, and no correlation was found for the USDA Pisum
core (r=0.01; Figure 2). The
complete data set and accessions are available through the internet (http://www.ars-grin.gov/npgs/)
or by contacting the curator (coynec@wsu.edu).
