Mutations at several loci suppress vegetative axillary meristem initiation in pea
Rameau, C., Bellec, Y., Grillot, P. Station de Genetique et d’Amelioration des Plantes
Inst. National de la Recherche Agronomique
Versailles, France
Parmenter, K.S., Beveridge, C.A. Dept. of Botany, The Univ. of Queensland
St Lucia, QLD, Australia
Turnbull, C.G.N. Dept. of Agric. Sci., Imperial College
Univ. of London, Wye, Kent, UK
The study of plant shoot architecture in pea has been mainly via the mutational analysis of increased branching phenotypes. These include the mutants fr, fru, ram, bushy and the well studied ramosus (rms) series (1, 3, 14). To date, the only reported mutations in pea that display a decreased branching phenotype are the pleiotropic photoperiod response mutants sn, ppd and dne, in which branching is indirectly decreased under short day conditions, probably as a result of altered assimilate partitioning (5, 6, 10) rather than mutation at loci which specifically promote branching.
In wild type peas, vegetative axillary meristems generally remain as small, inhibited buds, although decapitation and short photoperiods can stimulate their release and outgrowth into lateral branches. The RMS model of branching control provides evidence for two novel signals that regulate the inhibition of shoot branching in intact plants (2, 3). The regulation of axillary meristem initiation is less well understood.
Six RMS loci have been identified from 33 rms branching lines (1, 7, 8, 13) and multiple mutant alleles are known for each locus. This amount of redundancy suggests that the number of RMS loci identified through mutagenesis may be approaching saturation. To advance the investigation of branching stimulation, we have undertaken a mutagenesis of two branching lines and screened for decreased branching phenotypes. We report here the first description of one class of mutants obtained from this mutagenesis, the sax (suppressed axillary meristems) mutants, which display reduced numbers of vegetative axillary meristems. Whereas the RMS genes control bud inhibition, SAX genes may control bud initiation.
5200 seeds of the rms3-4 line, T2-30, and 5200 seeds of the rms4-3 line, M3T-946, were treated with EMS as described by Rameau et al. (7). These M1 seeds were sown for selfing and M2 seeds were harvested from 3831 M1 plants for T2-30 and 4267 M1 plants for M3T-946. These M2 progenies were harvested individually. For the screening, 14 seeds per family were sown in trays and plants showing decrease branching were replanted in 2-L pots for bulking and confirmation of the phenotype in the next generation. Mutant lines were described according to the initial genotype; revA lines originated from rms4-3 seed and revB lines from rms3-4 seed. Planting conditions for allelism and inheritance tests at Versailles were as described by Rameau et al. (7), with plants being grown under a 16-h photoperiod.
Photoperiodic characterisation of the mutant lines was conducted in controlled environment cabinets within a glasshouse at Imperial College, Wye. Plants were sown, two per pot, in 2-L pots containing a peat/sand/perlite (1:1:1) compost mix and were supplied weekly with liquid fertiliser. Temperature was 22°C day, 18°C night. All plants received 8 h natural light per day, supplemented by Son-T lamps for the first 3 weeks. Long day extension to give a 16-h photoperiod was provided by incandescent tungsten lamps.
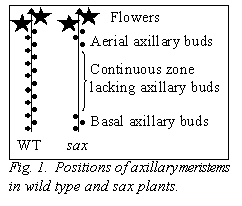
During the screening, several plants were kept because they displayed decreased branching due to empty axils in a continuous series from the third or fourth node until a few nodes below the flowering node. Consequently, all of these mutant lines displayed >10 budless axils per plant in a large central zone of the shoot (Fig. 1 and Table 1), whereas Térèse (cultivar of the initial lines rms3-4 and rms4-3, le af) plants displayed an average of 1.5 ± 0.3 budless axils per plant (n = 20). In at least lines revA-1102, revA-3166 and revB‑1208, the numbers of budless axils and aerial axils with buds were increased under short days (Table 1). The same number of budless axils was observed in the sax mutants on both Térèse and mutant rms3 or rms4 backgrounds (data not shown). Fewer branches tended to grow out from the basal nodes where a meristem was present in the mutant revA‑3166 when compared with its initial line rms4-3, M3T-946 (Fig. 2A). Once released, the length of the basal branches was not altered (Fig. 2B).

Table 1. Phenotype of sax lines under long (16h) and short (8h) photoperiods. NFI, node of floral initiation; Té, Térèse; s.e., standard error. n = 4-8.
|
|
|
|
Short days |
|||||||||||
|
No. nodes bearing buds |
|
No. nodes bearing buds |
NFI |
|||||||||||
|
|
|
|
|
|||||||||||
|
mean |
s.e. |
mean |
s.e. |
mean |
s.e. |
mean |
s.e. |
mean |
s.e. |
mean |
s.e. |
|||
|
Térèse |
RMS |
buds throughout* |
23 |
0.4 |
buds throughout* |
27 |
0.3 |
|||||||
|
Té x revA- 1102 |
RMS |
3.0 |
0.0 |
4.4 |
0.7 |
23 |
0.0 |
2.7 |
0.2 |
6.7 |
0.5 |
29 |
0.3 |
|
|
Té x revB- 1208 |
RMS |
3.6 |
0.5 |
4.3 |
1.2 |
20 |
0.4 |
3.7 |
0.2 |
5.9 |
1.6 |
27 |
0.5 |
|
|
M3T-946 |
rms4 |
buds throughout* |
19 |
0.3 |
buds throughout* |
25 |
0.4 |
|||||||
|
revA-3166 |
rms4 |
2.1 |
0.1 |
2.4 |
0.2 |
22 |
0.2 |
2.3 |
0.0 |
4.6 |
0.6 |
30 |
0.8 |
|
*occasional bare axil
F1 hybrids between the mutant lines revA-1102, revA-3166, revA-3367, revA‑3458, revA-3552 and revB-1208 and cv. Térèse were restored to a SAX or near SAX phenotype (Table 2), with a continuous or near continuous series of axillary buds along the stem. In some instances, incomplete dominance was displayed by heterozygous segregants (for example, in the F2 (Térèse x revA-1102) population of back-cross 1 as given in Fig. 3). Similar results have been observed for the lines revA-3166, revA-3367, revA-3458, revA‑3552 and revB-1208 (data not shown). Those heterozygous segregants that had a high number of budless axils usually displayed those axils in a discontinuous manner, whereas the sax mutant segregants produced a continuous series of budless axils. The observed F2 numbers were in good agreement with a 3 normal:1 mutant ratio (Table 2). Mutant-type F2 segregants bred true in the F3. Thus, we have concluded the six mutant lines show single-gene, partially recessive inheritance.
Table 2. Results of crosses testing inheritance. WT, wild type; M, mutant.
|
|
|
||||
|
Mutant line |
F1 |
F2 segregation |
Chi-square |
Probability |
|
|
WT |
M |
testing 3:1 |
|||
|
|
|
|
|
|
|
|
BC1 (Térèse x revA-1102) |
WT1 |
47 |
17 |
0.08 |
0.77 |
|
BC1 (revA-3166 x Térèse) |
WT1 |
35 |
10 |
0.19 |
0.67 |
|
BC1 (Térèse x revA-3667) |
WT1 |
27 |
8 |
0.30 |
0.57 |
|
BC1 (revA-3458 x Térèse) |
WT1 |
12 |
2 |
0.85 |
0.35 |
|
revA-3552 x Térèse |
WT1 |
15 |
5 |
0.00 |
1.00 |
|
Térèse x revB-1208 |
WT1 |
35 |
7 |
1.56 |
0.21 |
1incomplete dominance of the WT phenotype in the heterozygote.
Allelism tests between the six lines revealed at least two new loci (Table 3). Mutant lines revA-1102 and revA-3552 were demonstrated to be allelic. This locus was designated SAX1, with type line revA-1102 (=sax1-1) and allele revA‑3552 (sax1‑2). Mutants revA‑3166, revA-3367 and revA‑3458 were not allelic with revA-1102 and were demonstrated to be allelic with each other. Line revA‑3166 (sax2-1) was designated as the type line for this second locus, SAX2, and lines revA‑3367 and revA-3458 as alleles sax2-2 and sax2-3 respectively. Mutant revB-1208 was not allelic with revA-1102.
A. 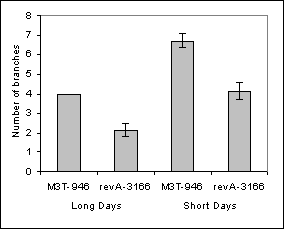 B.
B.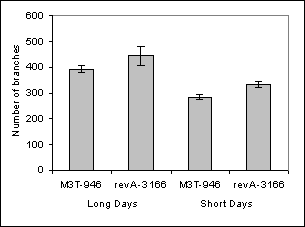
Fig. 2. Comparison of basal branching (nodes 1-2) of mutant line revA-3166 (sax2-1) and its initial line rms4-3, M3T-946. A. Number of basal branches >100mm per plant, B. Average length of basal branches. Plants were scored 77 days after planting. Error bars indicate standard error.
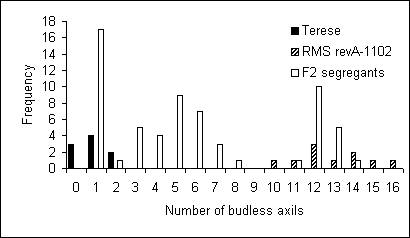
Fig. 3. Distribution of the number of budless axils on the main stem for Térèse (black), the mutant line revA-1102 on a Térèse RMS background (black and white) and the F2 segregates of a cross between these lines (white).
Table 3. Results of crosses testing for allelism between lines. A, allelic; NA, not allelic, no entry, cross not done.
|
|
revA-1102 |
revA-3552 |
revB-1208 |
revA-3166 |
revA-3367 |
|
revA-3552 |
A |
- |
|
|
|
|
revB-1208 |
NA |
|
- |
|
|
|
revA-3166 |
NA |
|
|
- |
|
|
revA-3367 |
NA |
|
|
A |
- |
|
revA-3458 |
NA |
NA |
|
A |
|
Mutant lines revA-1102 and revA-3552 were non-pleiotropic. Compared with the usual afila phenotype of Térèse and the initial line M3T-946, mutants revA-3166, revA‑3367 and revA-3458 all had a “bunched tendrils” phenotype (Fig. 4). This tendril phenotype was not confined to the budless axils, but rather was extended for the full length of the stem. The tendril phenotype exhibited 100% co-segregation with the 19 mutant-type plants in the 71 plant F2 population (revA-3166 x Térèse). F1 hybrids with the lines revA‑3166, revA-3367 and revA-3458 exhibited the Térèse af phenotype rather than the “bunched tendrils” phenotype of the mutant. Moreover, it has been obtained for 3 independent lines mutated in the same gene (see below). The mutant revB-1208 appeared mildly pleiotropic, with decreased fertility and slightly altered stipule colour and shape.

Reported here are seven sax lines obtained from the EMS mutagenesis of the two branching lines rms3-4, T2-30, and rms4-3, M3T-946. Line revA-1102, designated sax1‑1, proved to be allelic with another mutant line (revA‑3552, sax1-2). A further three lines (revA-3166, revA-3367 and revA-3458) proved to be mutations of a second SAX locus, SAX2. The seventh sax line, revB-1208, was not allelic with sax1 and the allelism test between revA-3166 (sax2-1) and revB-1208 has yet to be completed. However, the presence of the “bunched tendril” phenotype in all three sax2 mutants and the absence of this phenotype in revB-1208 argue for the existence of a third sax locus.
The sax series of mutants provide the first opportunity to investigate vegetative axillary meristem initiation in pea. It appears that the genes already identified act during different stages of phytomer development, with SAX2 probably required at an earlier point in development than SAX1, because of the function of SAX2 in tendril development. The mutations also appear to differentially affect zones of the main stem (Fig. 1 and Table 1). As well as the lack of axillary meristems at the central nodes, bud outgrowth at basal nodes appears to be altered in the mutant revA-3166, as indicated in Fig. 2. The number and developmental timing of commencement of the aerial buds was modulated by photoperiod (Table 1). This suggests that the capacity to produce aerial axillary meristems may be associated with the transition from vegetative to reproductive growth. It is interesting to note that photoperiod response is conferred in part by genes that regulate long-range signals (e.g., Sn, Dne, Ppd). The sax mutants will be useful to investigate the relationship between the flowering genes, photoperiod, long-distance signals (both for branching and flowering), bud outgrowth and axillary meristem development. The phenotype of the sax pea mutants is somewhat similar to the phenotype of the lateral suppressor and blind mutants of tomato (4, 16) and the revoluta mutant of Arabidopsis (15). The Ls, Bl and REV genes have recently been cloned using positional cloning (9, 11, 12, 17) and provide good candidate genes for the cloning of SAX pea genes despite the sax mutants differing in that they do not appear to have altered flower or inflorescence development.
In addition to their value for basic research, the sax mutants may also prove useful in the generation of improved architecture in agricultural cultivars. These lines have the capacity for strong basal branching without the undesirable profuse aerial branching present in most rms mutants.
1. Arumingtyas, E.L., Floyd, F.S., Gregory, M.J. and Murfet, I.C. 1992. Pisum Genetics 24:.17-31.
2. Beveridge, C.A. 2000. Plant Growth Regul. 32: 193-203.
3. Foo, E., Turnbull, C.G.N. and Beveridge, C.A. 2001. Plant Physiol. 126: 203-209.
4. Mapelli, S. and Kinet, J.M. 1992. Plant Growth Regul. 11: 385-390.
5. Murfet, I.C. and Reid, J.B. 1985. In: Hebblethwaite, P.D., Heath, M.C. and Dawkins, T.C.K. (eds.). The pea crop: a basis for improvement. Butterworths, London, pp 67-80.
6. Murfet, I.C. and Reid, J.B. 1993. In: Davies, D.R. and Casey, R. (eds.). Peas—Genetics, Molecular Biology and Biotechnology. CAB International, Wallingford, UK, pp 165-216.
7. Rameau, C., Bodelin, C., Cadier, D., Grandjean, O., Miard, F. and Murfet, I.C. 1997. Pisum Genetics 29:.7-12.
8. Rameau, C., Murfet, I.C., Laucou, V., Floyd, R.S., Morris, S. and Beveridge, C.A. 2002. Physiol. Plant. 115: 458-467.
9. Ratcliffe, O.J., Riechmann, J.L. and Zhang, J.Z. 2000. Plant Cell 12: 315-317.
10. Reid, J.B. and Murfet, I.C. 1984. Ann. Bot. 53: 369-382.
11. Schmitz, G., Tillmann, E., Carriero, F., Fiore, C., Cellini, F. and Theres, K. 2002. Proc. Natl. Acad. Sci. 99: 1064-1069.
12. Schumacher, K., Schmitt, T., Rossberg, M., Schmitz, G. and Theres, K. 1999. Proc. Natl. Acad. Sci. 96: 290-295.
13. Symons, G.M and Murfet, I.C. 1997. Pisum Genetics 29: 1-6.
14. Symons, G.M, Murfet, I.C., Ross, J.J., Sherriff, L.J. and Warkentin, T.D. 1999. Physiol. Plant. 107: 346-352.
15. Talbert, P.B., Adler, H.T., Parks, D.W. and Comai, L. 1995. Development 121: 2723-2735.
16. Williams, W. 1960. Heredity 14: 285-296.
17. Zhong, R. and Ye, Z.-H. 1999. Plant Cell 11: 2139-2152.