Observation of a phenomenon resembling hybrid dysgenesis,
in a wild pea subspecies Pisum sativum
ssp. elatius
Bogdanova, V.S. and Berdnikov, V.A.
Inst. of Cytology and Genetics, Russian Academy of Sciences
Novosibirsk, Russia
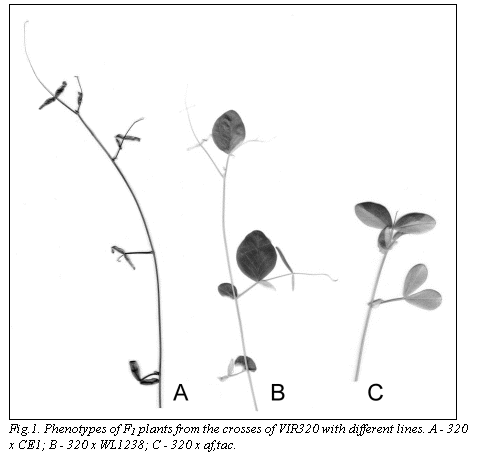 In a series of crosses of wild peas with cultivated
forms we found that one line, VIR320, was peculiar in that the characters of hybrid
plants produced when VIR320 was used as a female parent differed from those when
it was used as the male parent. This line has been assigned to P.
sativum ssp. syriacum (which
is a synonym of P. sativum ssp. elatius
var. pumilio). It originated from Palestine and was collected on
an early expedition of N.I.Vavilov.
When VIR320 was used as the
female parent in crosses, the
majority of the F1
plants were abnormal to some extent. Hybrid
plants pro-duced from the reciprocal
cross were normal. Observed
abnormalities included chloro-phyll pigmentation
(sectors of chlorotic tissue on the
normal background and vice versa), reduced leaves, and sterile flowers (Fig.1).
In a series of crosses of wild peas with cultivated
forms we found that one line, VIR320, was peculiar in that the characters of hybrid
plants produced when VIR320 was used as a female parent differed from those when
it was used as the male parent. This line has been assigned to P.
sativum ssp. syriacum (which
is a synonym of P. sativum ssp. elatius
var. pumilio). It originated from Palestine and was collected on
an early expedition of N.I.Vavilov.
When VIR320 was used as the
female parent in crosses, the
majority of the F1
plants were abnormal to some extent. Hybrid
plants pro-duced from the reciprocal
cross were normal. Observed
abnormalities included chloro-phyll pigmentation
(sectors of chlorotic tissue on the
normal background and vice versa), reduced leaves, and sterile flowers (Fig.1).
We
made crosses between VIR320 and 47 accessions, using the former as the female parent. The number of F1
plants obtained
from each cross varied from 1 to 15.
Of the alternate
parents, 32 were from
non-cultivated acces-sions and 15 from
cultivated types. In only six cases (L100,
VIR2521, JI1794, L99, P008 and WT301) abnormalities were not observed in the F1 generation, and in all these
cases the alternate parent represented
a wild form of
P. sativum
or P. fulvum (WT301). The degree of abnormality
observed varied, depending on the accession used as the pollen parent. There was a positive correlation
between the degree of the abnormality and the fraction
of F1 plants exhibiting an abnormality, with the frequency of
abnormal plants varying between 0 and 20%.
It proved useful to divide all accessions into two groups, those 'compatible' with VIR320
(producing only normal progeny in crosses) and those
'incompatible' with VIR320 (producing abnormal progeny in
crosses). The phenotypes resulting
from crosses with these two types of alternate parent are schematically
presented in Fig.2. One of the accessions that caused the most severe anomalies
in the progeny was VIR1451 (Arkhangelsk region) which was used as a tester in
the experiments described below.
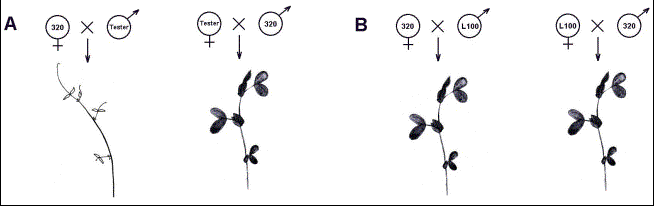
Fig.2.
Schematic model of phenotypes observed in seedlings from crosses with
incompatible (A) and compatible (B) lines.
We
investigated the mode of inheritance of the ability of line VIR320 to induce
aberrations in the progeny using two types of crosses: 1) crosses with incompatible lines; 2) crosses
with compatible lines.
We investigated segregation in
F2, and the trait we scored was the ability of an F2 plant to produce
aberrant progeny when pollinated with foreign pollen. In
the presentation of results we use the ♀ and ♂ symbols to denote maternal and paternal lines,
respectively. The line VIR320 is abbreviated to 320.
In all crosses, the maternal
parent is indicated first.
Crosses with incompatible lines
 Each plant from an F2 population of 120 plants derived from the cross WL1238 x 320 was pollinated with VIR1451. None of the 821 plants produced
from these crosses was phenotypically abnormal. We can summarize these results in the following
scheme:
Each plant from an F2 population of 120 plants derived from the cross WL1238 x 320 was pollinated with VIR1451. None of the 821 plants produced
from these crosses was phenotypically abnormal. We can summarize these results in the following
scheme:
F2[♀
x
320] x
VIR1451 —> normal
When
VIR320 plants were pollinated using 43 F2 plants from the cross
WL1238 x 320 the F2 plants appeared
to resolve into three classes (Table 1), one that produced only abnormal
progeny, one that produced both normal and abnormal progeny, and one that
produced only normal progeny.
It appears that in incompatible crosses either the presence of VIR320
genes in the pollen or the presence of VIR320 cytosol is the factor responsible
for the appearance of the observed abnormalities.
Crosses with compatible lines
In
this type of cross the ability of VIR320 to induce aberrations in the progeny
was approximately the same regardless of the direction of the cross
(Table 2). The line L100 was used as the compatible parent.
Table 2. Results of pollinations of
F2 plants from compatible crosses with VIR1451 pollen.
|
F2
population
|
N
|
Number
of F2 producing
|
|
Only
abnormal progeny
|
Both
abnormal and normal
|
Only
normal progeny
|
|
320
x
♂
|
29
|
04
(12 progeny)
|
13
0 (44 norm. + 22 abn.)
|
12
0 (61 progeny)
|
|
♀ x
320
|
52
|
14
(72 progeny)
|
24
(100 norm. + 98 abn.)
|
14
(115 progeny)
|
In
the experiment with the ♀ x 320 F2 population, the segregation of phenotypes is very
close to a 1:2:1 ratio,
suggesting that there is one (or
several closely linked) nuclear factor responsible for aberrations observed.
We conclude that the ability of the line VIR320 Pisum sativum ssp. elatius to cause aberrations in the
progeny is determined by the nuclear genome, but this effect can
be observed only in the presence of compatible cytoplasm. Abnormal progeny occurs in crosses where maternal plants refer
to the line VIR320 or its hybrids with compatible lines.
Phenotypic aberrations observed by
us are very similar to those described by Lutkov (4) in distant hybrids of pea.
However, in experiments of Lutkov, abnormal plants were observed in F2
and F3 generations, while we could observe the phenomenon only in the F1 after pollination of VIR320 (or its hybrids) with a tester
pollen.
There are some types of crosses
where phenotypes of the resulting progeny depend on the direction of the cross.
For example, cytoplasmic male sterility (CMS), manifested as
inability to produce fertile pollen or functional anthers, is maternally inherited although it is influenced by nuclear genes. CMS
is commonly associated with changes in mitochondrial gene expression (reviewed
in 6). In fact, VIR320 line acts like a source
of sterility-inducing cytoplasm, whereas compatible lines perform as if carrying
nuclear fertility restorer genes. However, segregation in the F2 of
compatible crosses indicates that we are dealing with a nuclear factor that can
be transmitted both maternally and paternally, although the source of cytoplasm
plays an important role. The issue appears to be involved in the phenomenon of
cytoplasmic incompatibility (CI) described for some arthropod species and
associated with endocellular bacteria of Wolbachia
species. CI is maternally transmitted and appears as reproductive aberrations
when an infected male is crossed with a female either devoid of Wolbachia
or harboring another strain of bacteria (see for example, 1). This aberration
can be overcome by antibiotic treatment against microbial invasions. We treated
seeds of VIR 320 and tester lines with doxycycline (25 μg/ml and 40 μg/ml). The
resulting seedlings were pale-green indicating that the treatment was effective;
however, the treatment did not abolish aberrations in the progeny.
Thus,
we suppose that the phenomenon observed most closely resembles hybrid dysgenesis
described in Drosophila (3). In flies,
the dysgenic syndrome appears in hybrid progeny when mobile genetic elements of
certain type come from the male parent and the hybrid genome conflicts with
cytoplasm of the oocyte produced by the female lacking active copies of this
mobile element. The resulting mobilization of the transposon in the hybrids
causes multiple genetic disorders including complete or partial sterility. It is
known that plant genomes may contain numerous copies of transposable elements
(5). By analogy with Drosophila, we
can suppose that the genomes of the majority of pea samples are saturated with
some type of transposons which are, however, inactive due to accumulation in the cytoplasm
of a repressor encoded on the DNA of the
mobile element. Male gametes lack the repressor or contain very little of it.
A cross involving two such lines does not cause dysgenesis because mobile elements delivered by the male gamete are
controlled by the repressor produced by transposons of the egg. We suppose that
the genome of the line VIR320 is devoid of mobile elements capable of repressor
production, therefore, when an egg of this line meets with a male gamete of an
incompatible line, transposons of the latter begin active transpositions and
repressor production. As for compatible wild samples, we suppose that their
genome harbors defective mobile elements not capable of active transposition but
producing a factor of repression which is accumulated in cytoplasm thus making
it non-reactive. Therefore, crosses of these lines with common testers produce
normal progeny, and the off-spring from the crosses with VIR320 also does not
suffer from genetic disorders caused by transpositions of mobile elements.
It
should be noted that crosses of Pisum
sativum with other pea taxa such as P. sativum ssp.
abyssinicum and P. fulvum are successful (with rare exceptions) only when P.
sativum is used as the female plant (2, 7). In
addition, an
F2 may segregate for aberrant plants (4)
similar to those described in the present study. It is possible, therefore, that
nuclear-cytoplasmic conflict contributes to interspecies isolation and, thus, to speciation.
Acknowledgement: This
work was supported by the Russian State Program 'Russian Fund for Fundamental
Research', grant No 99-04-49970.
1. Bourtzis,
K., Androniki, A., Markakis, G. and Savakis, C. 1996. Genetics 144:
1063-1073.
2.
Dyachuk, P.A. 1975. In:
Khvostova, V.V. (ed.) Genetics and Selection of Pea,. Novosibirsk. Nauka, pp
197-223 (in Russian).
3. Engels,
W.R. 1979. Genet. Res. 33:
219-236.
4. Lutkov,
A.N. 1930. Proceedings of All-Union Congress on Genetics, Selection and
Seed Breding, Leningrad, 1929-30, Vol. 2, p.353-367 (in Russian).
5. Pearce,
S.R., Harrison, G., Li, D., Heslop-Harrison, J.S., Kumar, A. and Flavell, A.J.
1996. Mol. Gen. Genet. 250: 305-315.
6. Schnable,
P.S. and Wise, R.P. 1998.
Trends Plant Sci. 3: 175-180.
7. Sobolev,
N.A. and Agarkova, S.N. 1975.
In: Khvostova, V.V. (ed.). Genetics and Selection of Pea, Novosibirsk.
Nauka, pp 141-160 (in Russian).
 In a series of crosses of wild peas with cultivated
forms we found that one line, VIR320, was peculiar in that the characters of hybrid
plants produced when VIR320 was used as a female parent differed from those when
it was used as the male parent. This line has been assigned to P.
sativum ssp. syriacum (which
is a synonym of P. sativum ssp. elatius
var. pumilio). It originated from Palestine and was collected on
an early expedition of N.I.Vavilov.
When VIR320 was used as the
female parent in crosses, the
majority of the F1
plants were abnormal to some extent. Hybrid
plants pro-duced from the reciprocal
cross were normal. Observed
abnormalities included chloro-phyll pigmentation
(sectors of chlorotic tissue on the
normal background and vice versa), reduced leaves, and sterile flowers (Fig.1).
In a series of crosses of wild peas with cultivated
forms we found that one line, VIR320, was peculiar in that the characters of hybrid
plants produced when VIR320 was used as a female parent differed from those when
it was used as the male parent. This line has been assigned to P.
sativum ssp. syriacum (which
is a synonym of P. sativum ssp. elatius
var. pumilio). It originated from Palestine and was collected on
an early expedition of N.I.Vavilov.
When VIR320 was used as the
female parent in crosses, the
majority of the F1
plants were abnormal to some extent. Hybrid
plants pro-duced from the reciprocal
cross were normal. Observed
abnormalities included chloro-phyll pigmentation
(sectors of chlorotic tissue on the
normal background and vice versa), reduced leaves, and sterile flowers (Fig.1).

