 ‘Sparkle’ and one from line
Slow. The five plants from each
accession were grown to maturity and seed collected for further study.
‘Sparkle’ and one from line
Slow. The five plants from each
accession were grown to maturity and seed collected for further study.
Allozyme analysis of Pisum sativum ssp. abyssinicum and the development of a genotypic definition for this subspecies
Weeden, N.F. and Montana State Univ., Bozeman, MT, USA
Wolko, B. Inst. of Plant Genetics, Polish Academy of Sciences, Poznan, Poland
The Abyssinicum pea, Pisum sativum ssp abyssinicum (A. Braun) Govorov (2) is easily recognized by its strongly serrate leaflets, particularly from nodes 5 to 12. As its name implies, the taxon was initially found in northern Africa in the region now forming Eritrea and Ethiopia. The serrate character was shown to be controlled by a dominant gene in crosses using the line 808 (= WBH 808) (11). Sutton initially gave the symbol Ser to this gene, but Wellensiek (14) referred to it as Td, and we will use the latter terminology. The subspecies appears to be intermediate between P. s. ssp. sativum and P. s. ssp. elatius. With the former, it shares the traits of indehiscent pod, relatively large and smooth seeds, and a relatively thin testa. However, P. s. ssp. abyssinicum also is dominant for A, recessive for up, and has relatively small flowers and pods, all characteristics that are fixed or known to occur in P. s. ssp. elatius and are often lacking in P. s. ssp sativum. According to Lamprecht (5), the line WBH 808 differs from the standard karyotype by two (1--7, 4--6) translocations. The precise chromosomes involved in these translocations need to be confirmed because Lamprecht based this conclusion on his mistaken assignment of Le to chromosome 4. However, we definitely observe partial sterility in the F1 and later generations from crosses between the Abyssinicum pea and numerous commercial lines of P. s. ssp. sativum (unpublished results).
Rosen (10) performed a detailed study of the inheritance of segregating characters in a P. s. ssp. sativum x P. s. ssp. abyssinicum F2 population. He developed the following genotype for the P. s. ssp. abyssinicum germplasm he used: Le, la or cry, td, d, Gp, pur, A, Ar, B, Am Wb, Fa, St, K, Tl, P, V, Bt, Cp, S. m, F, I, R, Pl, U. (Note that Rosen used td rather than Td to define the serrate trait because he believed that the trait was incompletely dominant.) Most of these alleles are relatively common in the pea germplasm and are not particularly diagnostic for P. s. ssp. abyssinicum. However, the combination A, Td, d, U is relatively rare in the pea germplasm and could be useful for identifying this subspecies if generally applicable. We undertook our study of the genetic diversity in P. s. ssp. abyssinicum to develop a more general genetic definition for the taxon as a whole. Here we present our findings based on a survey of the genetic variation at 48 loci in pea accessions displaying strongly serrate leaflets.
Materials and Methods
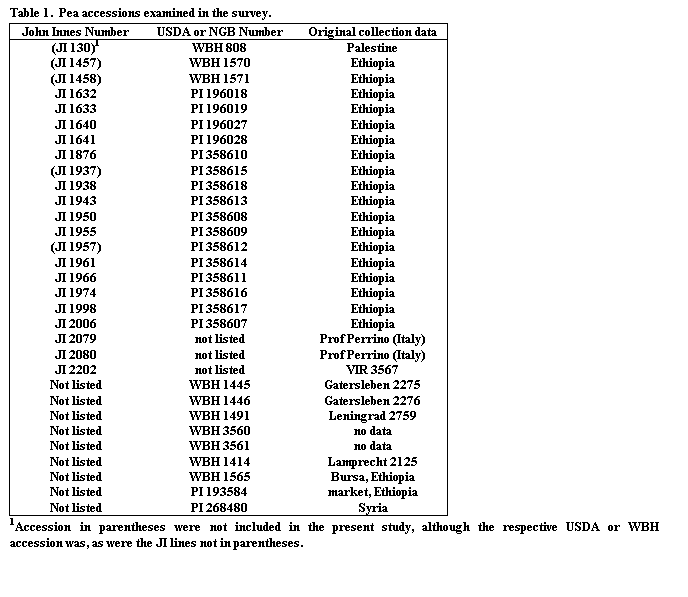
A total of 41 accessions labeled as P. s. ssp. abyssinicum were analyzed. These were obtained from the USDA collection at Geneva, NY (now at Pullman WA) (16 accessions), from the John Innes Institute, Norwich, UK (17 accessions), and from Wiatrowo, Poland (8 accessions, with Nordic Genebank numbers) (Table 1). Many of the John Innes lines we examined originally came from the USDA collection and represent duplicate samples of this germplasm. Thus, the material we examined actually represented a maximum of 27 distinct P. s. ssp. abyssinicum accessions. Four additional lines (WBH 1414, WBH 1565, PI 193584 and PI 268480) were also included in the study because they displayed sharply serrate leaflets, although they differed in one or more ways from the normal P. s. ssp. abyssinicum phenotype. W1414 is the type line for the Ser gene as defined by Lamprecht (4) and has white flowers. W1565 has violet flowers but also displays the Dco phenotype, which is atypical, although not unknown, in P. s. ssp. abyssinicum accessions. PI 193584 was collected in Ethiopia and consisted of white-flowered plants, some with the sharply serrate leaflets as well as some without this characteristic. PI 268480 is a P. s. ssp. elatius accession, a derivative of which was used by Polans (7) in his mapping of the gene responsible for leaflet serration.
Ten
seed were germinated from each accession, and each plant was scored for 18
morphological characters (Table 2). Plants
were initially grown in the glasshouse under long-day (16 hr day length, with
fluorescent and incandescent lighting supplementing natural daylight as
necessary). Five plants from each accession
were subjected to allozyme analysis on horizontal starch gels using 19 enzyme
assays, which reveal products of 30 loci. Allozyme
analysis was performed as described in Wendel and Weeden (15).
P. sativum ssp. sativum
controls were included on nearly every gel, usually consisting of an extract
from cv.  ‘Sparkle’ and one from line
Slow. The five plants from each
accession were grown to maturity and seed collected for further study.
‘Sparkle’ and one from line
Slow. The five plants from each
accession were grown to maturity and seed collected for further study.
Scoring of seed characters was performed both on the seed received from the germplasm collections and on the seed collected from the five plants. Two to five seed of this next generation were planted in the glasshouse under short-day conditions (12 hr day length) and the plants used to obtain confirming data as well as for analysis of sensitivity to photoperiod. During this second grow out, a powdery mildew infestation occurred naturally, and all accessions were scored for susceptibility to this disease.
Table 2. The 48 loci and their linkage group assignments on the pea linkage map
|
Linkage group |
Morphological and physiological loci |
Isozyme loci |
|
I |
D, I |
Alatc, Idh |
|
II |
A, Lf1 |
Aatp, Fum, Pgmp |
|
III |
Dpo, Le, M, Np, Rb |
Aatc, Acp3, Gal3, Lap1, Lap2 |
|
IV |
Fa |
Alatp |
|
V |
Bt, Fs, R, U |
Acp1, Est4, Nag, Pgdc, Px1 |
|
VI |
Er1, Gty, Pl |
Acp4, Prx3 |
|
VII |
Sn |
Aatm, Aldo, Amy, Est2, Gal2, Pep3, Pep4, Pgdp, Pgmc, Skdh, Sod |
|
Unmapped |
|
Pgk |
1Only alleles Lf and lf could be distinguished.
Very little polymorphism was observed within P. s. ssp. abyssinicum for the 48 loci scored. The only difference observed between USDA and John Innes samples representing the same accessions was that PI358608 displayed a d/Dco polymorphism, whereas all JI 1950 plants were Dco. From the over 2000 locus x accession combinations examined, within-line polymorphism was observed only in four cases (Dco in PI358608, Px1 in WBH 1445, and Fs/U in PI 358607 and JI 2080). Loci that exhibited polymorphism among the P. s. ssp. abyssinicum accessions are given in Table 3. Except for these loci, a consensus genotype for the subspecies, consisting of alleles invariant in our sample of the germplasm, can be assembled (Table 4). The accessions consistently formed an initial flower about node 14, regardless of daylength. Hence, we suggest a genotype of Lf, sn for two important flowering loci. Unique alleles were found at the loci Acp1 and Pgk. Both allozymes were fixed in all accessions of P. s. ssp abyssinicum but were not observed in any of the 250 pea accessions we had examined previously (12). Alleles at the remaining loci were found outside the taxon, although some, particulary those at Aatp, Fum, and Amy (see Discussion), were of limited distribution in the general collection.
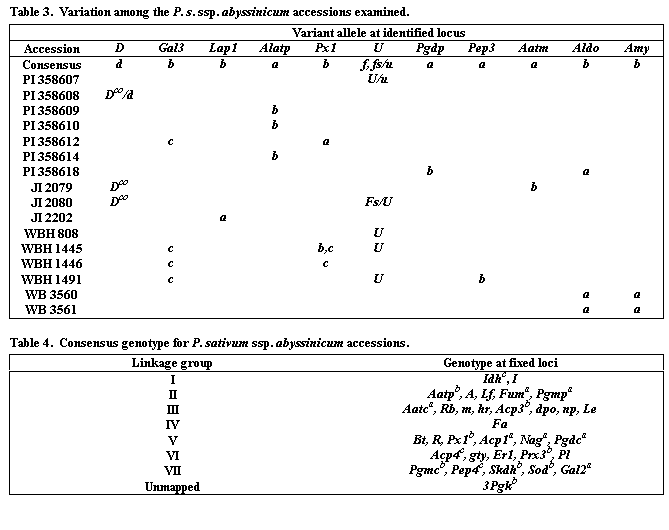 The four accessions that possessed serrate leaflets but differed from the
standard morphological type for P. s.
ssp. abyssinicum did not correspond well to the consensus genotype.
All four lines lacked the unique Acp1 and Pgk alleles, and only the P. sativum
ssp. elatius possessed the Aatpb or the Amyb allele.
However, this latter line differed from the P. s. ssp. abyssinicum
accession by several morphological characters (M, pl, Dpo, Sn) and the serration was slightly less
pronounced. WBH 1414 also lacked
black pigment in the hilum and differed at nine other isozyme loci from the
consensus genotype. Although WBH
1565 was not as morphologically divergent as WBH 1414, it still differed from
the consensus genotype at nine loci. PI
193584 also differed from the consensus genotype by a minimum of nine loci,
although the mixture of genotypes in that accession made an exact comparison
difficult.
The four accessions that possessed serrate leaflets but differed from the
standard morphological type for P. s.
ssp. abyssinicum did not correspond well to the consensus genotype.
All four lines lacked the unique Acp1 and Pgk alleles, and only the P. sativum
ssp. elatius possessed the Aatpb or the Amyb allele.
However, this latter line differed from the P. s. ssp. abyssinicum
accession by several morphological characters (M, pl, Dpo, Sn) and the serration was slightly less
pronounced. WBH 1414 also lacked
black pigment in the hilum and differed at nine other isozyme loci from the
consensus genotype. Although WBH
1565 was not as morphologically divergent as WBH 1414, it still differed from
the consensus genotype at nine loci. PI
193584 also differed from the consensus genotype by a minimum of nine loci,
although the mixture of genotypes in that accession made an exact comparison
difficult.
Discussion
Our results indicate that P. s. ssp. abyssinicum is a compact, well delineated taxon with a narrow genetic base and little allozyme variation. Several other researchers have included this subspecies in surveys of seed proteins (3, 9), isozymes (8, 12) and retrotransposons (6). In all these surveys, the Abyssinicum pea has formed a relatively distinct group, indicating that it is not just the Td allele that defines this taxon. Indeed, the Abyssinicum pea is sufficiently distinct that Braun (1) originally gave it specific status (P. abyssinicum). Our study confirms and extends these previous results in that we surveyed more accessions and more loci than previously investigated, as well as examining multiple plants from each accession.
Winfield and Green (15) emphasized the utility of genetics in the classification of pea cultivars but did not attempt to pursue such applications at the subspecific level. Here we have shown that P. s. ssp. abyssinicum can be clearly defined on the basis of genotype. At least three alleles (Td, Acp1a, and Pgkb) are highly diagnostic for the taxon, and the genotypic combination dpo, Td, m, Pl easily separates this taxon from all others recognized in the genus. We chose the dpo, Td, m, Pl combination as the most diagnostic rather than the A, Td, d, U combination mentioned earlier because neither d nor U were fixed in P. s. ssp. abyssinicum. JI 1950, JI 2079 and JI 2080 all display the Dco phenotype, and the first of these is listed as P. sativum ssp. sativum in the John Innes catalogue. However, all three of these accessions definitely cluster with other Abyssinicum pea accessions. Similarly, only five accessions (PI 358607, JI2080, WBH 808, WBH 1445 and WBH 1491) displayed the solid violet testa characteristic of U. The more common phenotype was a grayish tan testa.
Despite the presence of serrate leaflet margins in WBH 1414, WBH 1565, PI 193584 and PI 268480, none of these accessions could be considered P. s. ssp. abyssinicum. Each of these differed from the consensus Abyssinicum genotype at eight or more loci. None of the accessions belonging to the Abyssinicum cluster differed by more than three alleles from the consensus genotype. The consideration of additional loci, investigated by others (3, 8, 9) but not employed in the current study, would further distance the subspecies from the rest of the Pisum germplasm. An issue that may influence the precise interpretation of the data is our inability to resolve alleles known to exist at several isozyme loci. Zimniak-Przybylska and Przybylska (17) were able to resolve 11 alleles at their Amy2 (identical to our Amy). We know we missed some alleles using horizontal starch gel electrophoresis, and lacking DNA sequence data we may have missed many more. Such errors would result in our combining categories and perhaps missing additional alleles unique to the Abyssinicum pea. Thus, P. s. ssp. abyssinicum may be more distinct (possess more unique alleles) than we have reported.
Our data further support the intermediate position of P. s. ssp. abyssinicum between P. s. ssp. sativum and P. s. ssp. elatius. The relatively large seeds, lack of dehiscent pods and lack of a dormancy mechanism (hard seededness), strongly suggest that P.s. ssp. abyssinicum has undergone partial domestication. The unique leaflet morphology and Acp1 and Pgk genotype of the subspecies indicate that this domestication occurred at least somewhat independently of the domestication leading to most European cultivars. The subspecies probably went through a severe bottleneck either during the initial domestication events or possibly when transported into northern Africa. However, the b allele for Aatp is commonly found in P. s. ssp elatius but rarely in commercial germplasm,. placing the Abyssinicum pea with the wild subspecies in this respect. The a allele for Fum is predominately found in land races and wild subspecies. Similarly, the b and c alleles of Amy are characteristic of land races from the Middle East (Turkey, Iran, Afghanistan, and Pakistan), whereas the a allele is common in European lines. The fixation of the Fuma allele and the near fixation of the Amyb allele in P. s. ssp. abyssinicum provides further evidence for the affinity between this taxon and the wild subspecies.
The low genetic diversity observed in P. s. ssp. abyssinicum may in part be due to a relatively small number of accessions available in germplasm collections. The number independent accessions we actually examined may be overestimated because some of the WHB accessions may be duplicates of USDA or John Innes accessions. We tried to identify all such cases, but passport data regarding the original collection site is limited for many of the accessions we studied. There remain at least six P. s. ssp. abyssinicum accessions at the John Innes Institute that we did not investigate. In addition, there may be germplasm growing in northern Africa that is not represented in germplasm collections. If our sample of the P. s. ssp. abyssinicum germplasm is incomplete, our conclusions may be premature. However, the accessions available to us were collected by several different botanists and from a wide geographical region (Israel to Ethiopia). Sixteen of the accessions could be distinguished using some combination of markers, implying at least 16 independent collections were made. Based on the data available, we must consider P. s. ssp. abyssinicum as an isolated and genetically unique taxon that may be a important source of genetic diversity for breeding applications once the semi sterility problem is overcome. In any event, one and probably only one accession of P. s. ssp. abyssinicum need be included in any core collection of P. sativum germplasm.
01. Braun, A. 1841. Flora oder allg. Bot. Zeitung 17: 258-272.
02. Govorov, L.I. 1930. Trudy Prikl. Bot. 24:407.
03. Hadacova, V., Turkova, V., Hadac, E. and Klozova, E. 1980. Biologia Plantarum 22: 7-16.
04. Lamprecht, H. 1962. Agri Hort. Gen. 20: 63-74.
05. Lamprecht, H. 1964. Agri Hort. Gen. 22: 56-148.
06. Pearce, S.R., Knox, M., Ellis, T.H.N., Flavell, A.J. and Kumar, A. 2000. Mol. Gen. Genet. 263: 898-907.
07. Polans, N.O. 1993. Pisum Genetics, 25: 36-39.
08. Przybylska, J. Blixt, S., Parzysz, H. and Zimniak-Przybylska, Z. 1982. Genet. Pol. 23: 103-121.
09. Przybylska, J., Hurich, J. and Zimniak-Przybylska, Z. 1979. Genet. Pol. 20: 517-528.
10. Rosen, G. von. 1944. Hereditas 30: 261-400.
11. Sutton, A.W.. 1914. J. Lin. Soc. Bot. 42: 427-434.
12. Swiecicki, W.K., Wolko, B., Apisitwanich, S. and Krajewski, P. 2000. Gen. Res. Crop Evol. 47:583-589.
13. Weeden, N.F. and Wolko, B. 1988. Measurement of genetic diversity in pea accessions collected near the center of origin of domesticated pea. IPBGR final report., Rome, 20 pp.
14. Wellensiek, S.J. 1925. Bibl. Genetica 2: 343-476.
15. Wendel, J.F. and Weeden, N.F. 1989. In: Soltis, D.E. and Soltis, P.S. (eds.). Isozymes in Plant Biology. Dioscorides Press, Portland. pp 5-45.
16. Winfield, P.J. and Green, F.N. 1986. In: Styles, B.T. (ed.) Infraspecific Classification of Wild and Cultivated Plants. Clarendon Press, Oxford, pp 317-330.
17. Zimniak-Przybylska, Z. and Przybylska, J. 1983. Pisum Newslett. 15: 60-61.