New
ramosus mutants at loci Rms1, Rms3 and Rms4
resulting from the mutation breeding program at Versailles.
Rameau,
C., Bodelin, C., Cadier, D., Station
de Genetique
Grandjean, O. and Miard, F. et
d’Amelioration des Plantes
Institut
National de la Recherche Agronomique
Rte
de St. Cyr, F-78027 Versailles, France
Murfet, I. C. Department
of Plant Science,
University
of Tasmania
GPO
Box 252-55, Hobart, Tas 7001, Australia
Modification of the architecture of a plant has important agronomic consequences. Through selection, many crop plants have become shorter, have shorter life cycles and are less branched. For some species, a previously determinate growth habit has been replaced by an indeterminate growth habit and vice versa (14). In pea, standing ability has been improved by a reduction in both plant height (the le mutation) and foliage (the af mutation) to generate the dwarf, semi-leafless cultivars (9) which now represent a high proportion of those currently grown in northern Europe.
The branching habit of the plant is one of the main components of its architecture. In pea, controlling the basal branching of the plant has agronomic importance as the number of branches bearing pods has a major influence on seed number (8, 10). Moreover, increased basal branching seems to give a more determinate habit to the plant; there are more stems but with less reproductive nodes per stem. Despite the agronomic importance of this character, few studies concerning branching in pea have been performed with a view to improving crop performance and yield stability. At INRA, Versailles, we have recently conducted a mutagenesis program to identify genes with a direct influence on apical dominance. From an agronomic point of view, mutations which moderately increase the number of basal laterals (secondary stems) may prove advantageous. However, any mutations which directly alter apical dominance are useful for basic research into the control of branching.
From analysis of mutants that branch extensively from both basal and aerial nodes, five rms (ramosus) loci have already been identified in pea (1, 2, 6) and the role of auxin and cytokinin in the branching of these rms mutants is under investigation (3, 4, 5). The use of such single-gene mutants will undoubtedly lead to a better understanding of the control of lateral bud initiation and subsequent growth, and of the role of phytohormones in the control of apical dominance. From such physiological studies, new screens can be developed to isolate more specific mutants that may otherwise be difficult to screen or isolate because they confer only a quantitative effect on branching.
We report here on the genetic analysis of five new ramosus mutants selected at Versailles because of their profuse branching phenotype. All five mutations proved to be at previously identified loci, two at Rms1, two at Rms3 and one at Rms4.
Materials and Methods
Terese, the initial line used in the mutagenesis program, is a French spring cultivar with a dwarf habit (le) and the afila (af) leaf form. Terese plants occasionally produce basal lateral branches (generally at node 2), but otherwise have a strong apical dominance. This cultivar has a typical wild-type (WT) flowering behaviour (late flowering with a quantitative delay under short days) and presumably has the flowering genotype of Lf Sn Dne Ppd hr (13).
Table 1. Details of three mutagenesis experiments (agent EMS) undertaken at INRA using the dwarf (le), semi-leafless (af) cultivar Terese as the initial line.
|
Experiment |
I |
II |
III |
|
Number of M1 seeds |
1600 (glasshouse) |
3000 (glasshouse) |
2000 (field) |
|
Number of M1 plants giving M2 seeds |
1075 |
885 |
320 |
|
Number of M2 seeds |
5500 |
1580 (field) |
At least 320 x 14 |
|
Number of seeds or families screened |
5500 M2 seeds |
1186 M3 families (14 seeds per family) |
320 M2 families (14 seeds per family) |
|
Mutant lines with a ramosus phenotype |
T2-30 |
M3T-884, M3T-946, M3T-988 |
M2T-32 |
Table 2. F2 segregation data for crosses between the five new ramosus mutants and their initial line Terese.
___________________________________________________________________________
Cross WT Mutant Chi-sq.(3:1) Probability
__________________________________________________________________________
BC1 (T2-30 x Terese) F2 37 14 0.32 >0.5
(M3T-884 x Terese) F2 27 7 0.35 >0.5
BC2 (M3T-946 x Terese) F2 36 9 0.60 >0.3
BC3 (M3T-988 x Terese) F2 55 26 2.17 >0.1
BC2 (Terese x M2T-32) F2 35 16 1.11 >0.2
___________________________________________________________________________
The mutagenesis treatment has been adapted from Wang et al. (16). Seeds of cultivar Terese were soaked over night in water at 4- C. The imbibed seeds were treated with ethyl methane sulphonate (EMS) solution (4 mM) for 4 h at room temperature, soaked a few minutes in a solution of sodium thiosulphate (0.2 M) for decontamination and finally washed for 1 h under running tap water. M1 seeds were sown either in glasshouse or field conditions (Table 1). The M2 progenies were harvested individually. Usually, 14 M2 or M3 seeds per family (Table 1) were sown in trays for screening in the glasshouse. Screens were performed on young seedlings with about 4 or 5 leaves (counting from the first scale leaf as leaf 1). Potential mutant plants with increased branching in comparison with Terese were then replanted in 2 or 4-L pots and the phenotype confirmed in the next generation.
At Versailles, plants were grown in a heated glasshouse under a 16-h photoperiod. The potting mix was 100% peat (Aquiland, Landiras, France). The natural daylength was extended (or supplemented during the day when necessary) with sodium lamps (SON/T AGRO 400W, Philips, Ivry/Seine, France). Nutrient solution (7) was dissolved in the water supply which was given through an automatic drip system as necessary. At Hobart, plants were grown 1 per 140-mm slimline pot (volume 1.8 L) under an 18-h photoperiod; further details are given by Symons and Murfet (15).
Table 3. The results of crosses testing the five new mutants for allelism with each other and with previously genotyped (1, 2, 6) ramosus mutants. A, allelic; NA, not allelic; no entry, cross not done.
|
M3T-884 |
M3T-988 |
M2T-32 |
T2-30 |
M3T-946 |
|
| WL5237 (rms1) |
A |
NA |
NA |
NA |
|
| WL5918 (rms1) |
A |
A |
|||
| Wt15236 (rms1) |
A |
||||
| K524 (rms2) |
NA |
NA |
NA |
NA |
NA |
| WL6042 (rms3) |
NA |
A |
A |
NA |
|
| K164 (rms4) |
NA |
NA |
NA |
NA |
A |
| Wt15244 (rms5) |
NA |
NA |
NA |
NA |
|
| M3T-988 |
A |
- |
|||
| M2T-32 |
NA |
- |
|||
| T2-30 |
A |
- |
|||
| M3T-946 |
NA |
NA |
NA |
- |
Inheritance of the five new rms mutants was tested by crossing each mutant with the initial line, Terese (Table 2). Allelism was tested (Table 3) by crosses among the mutant lines and by crosses with representative lines for rms1 (WL5237, WL5918 and Wt15236), rms2 (K524), rms3 (WL6042), rms4 (K164) and rms5 (Wt15244).
Results
Three separate mutagenesis experiments (Table 1) were conducted using a total of 6600 seeds. Only 2280 of these M1 seeds gave M2 seeds, mainly because of poor light conditions in the glasshouse and poor sowing conditions in field. In the first experiment, where an average of 5 M2 seeds were obtained per M1 plant, all the M2 seeds were sown for screening. In the second experiment, less than 2 M2 seeds were obtained per M1 plant. Consequently, for this experiment the M2 seeds were sown in the field to obtain a better yield of seeds per family, and the screen was performed on the M3 generation. In the third experiment, the M1 seeds were sown in the field and 320 M2 families were screened in the glasshouse.
The five ramosus mutants selected during the different screens (Table 1) are all shorter than the WT and show profuse branching at both basal and aerial nodes well prior to the appearance of open flowers. The stems are thinner, and the orientation of basal laterals is more vertical, than for Terese. This phenotype has been confirmed in the subsequent generations. In crosses, mutant plants were clearly recognisable under the conditions used at both Versailles and Hobart.

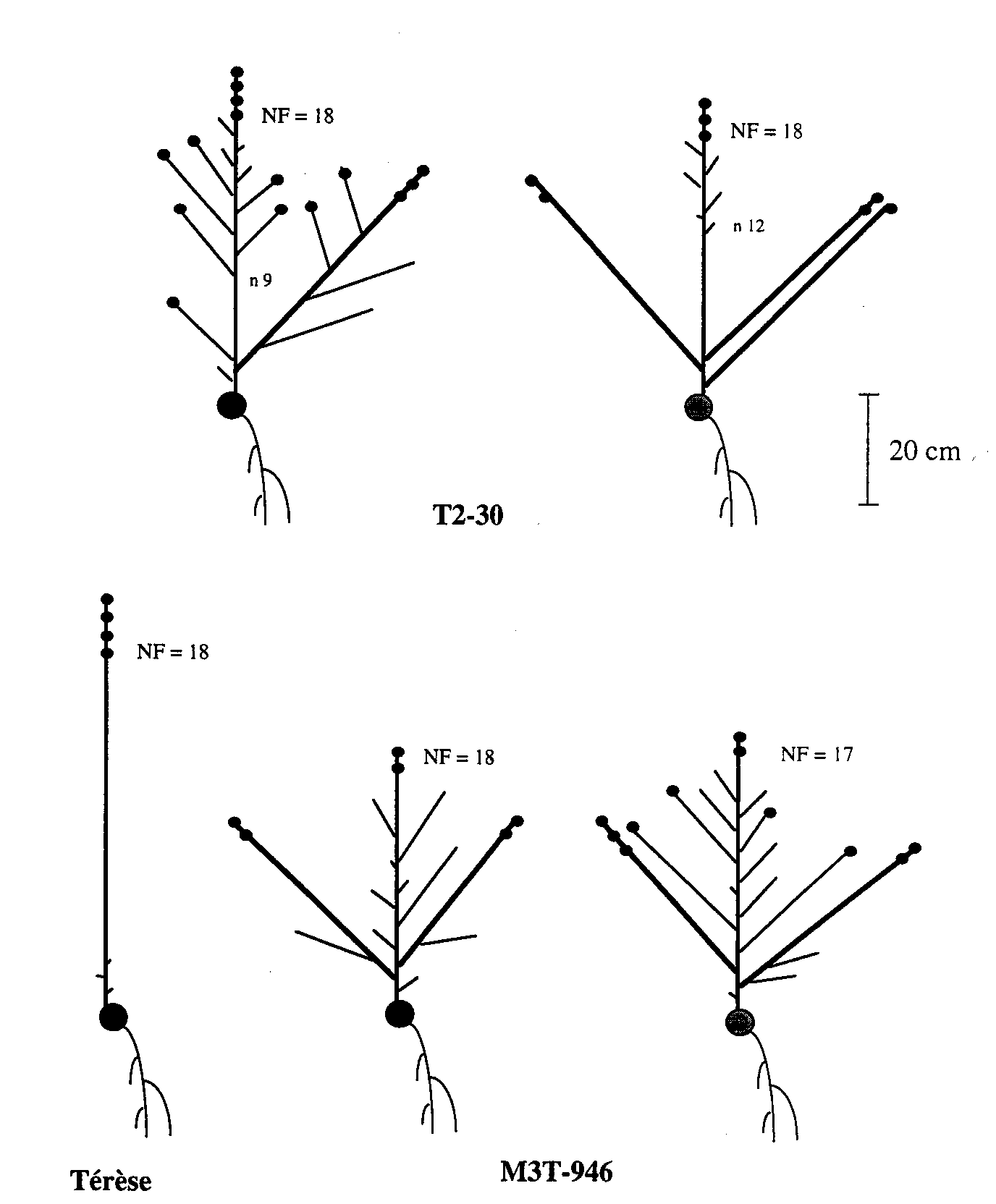
Fig.1. Diagrams of representative mature plants of the initial line Terese and mutant lines T2-30 (rms3-4) and M3T-946 (rms4-3). Shoot lengths, but not internode lengths, are drawn to scale. Small solid circles represent pod-bearing nodes and the large solid circle the cotyledonary node. Node of first flower (NF) is indicated for each plant and node of origin of the first aerial lateral (n 9, n 12) for the T2-30 plants. T2-30 displays a gap (G) branching pattern and M3T-946 a complete (C) branching pattern according to the classification scheme of Arumingtyas et al. (2). The plants were grown one per 2-L pot under a 16-h photoperiod.
Crosses between the mutants and the initial line Terese produced F1 plants with a WT phenotype. For each mutant line, the F2 generation showed a clear separation into normal and mutant types in good accordance with a 3:1 ratio (Table 2). Thus the five mutant lines showed single gene recessive inheritance.
The results of allelism tests (Table 3) demonstrate that mutants M3T-884 and M3T-988 are allelic with rms1 (alleles rms1-10, rms1-11), T2-30 and M2T-32 are allelic with rms3 (alleles rms3-4 and rms3-5), and M3T-946 is allelic with rms4 (allele rms4-3). [A table listing details of all reported mutants for loci Rms1 through Rms5 and suggested allele numbers is given by Symons and Murfet (15)].
Fig. 1 gives a precise description of representative mature plants of Terese (WT), T2-30 (rms3-4) and M3T-946 (rms4-3) grown individually in 2-L pots under 16-h glasshouse conditions. Mutant rms4-3 plants are slightly shorter than rms3-4 plants and both are shorter than Terese. The branching pattern shown by the rms3-4 and rms4-3 mutations in the dwarf Terese background under 16-h conditions at Versailles, is the same, respectively, as the branching pattern given by mutations rms3-1 (K487) and rms4-1 (K164) in a tall Torsdag background under 16-h conditions at Hobart (2). Line T2-30 plants have a gap (G) phenotype with a region devoid of branches between node 4 and nodes 8 or 9, while M3T-946 plants have a complete (C) branching phenotype.
F1 plants and parental controls for the cross Terese + M3T-946 (rms4-3) were grown at both Versailles and Hobart. At both locations the WT Rms4 allele was fully dominant for the trait ‘length of main stem’. Under Hobart conditions allele Rms4 was also fully dominant for branching habit: M3T-946 plants branched at both basal and aerial nodes while the Terese and F1 plants did not branch. In contrast, under Versailles conditions allele Rms4 showed only partial dominance for branching habit as 9 out of 10 F1 plants produced basal laterals compared with only 2 out of 9 Terese plants; all M3T-946 plants produced both basal and aerial laterals. We suggest that triggering of lateral bud release is a threshold phenomenon and that the slightly shorter photoperiod used at Versailles (16 versus 18 h) has altered the internal hormonal situation sufficiently to expose the partial dominance of Rms4 over rms4-3. The shorter photoperiod would lead to increased activity of the genes (Sn, Dne and Ppd) in the photoperiod response pathway (11, 13). Greater output of flower inhibitor from this pathway would in turn increase the tendency for basal lateral outgrowth (2, 12, 13).
Discussion
From 2280 M1 plants treated with EMS, five new ramosus mutants were obtained from the dwarf cultivar Terese. Two (M3T-884, M3T-988) proved to be allelic with rms1, two (T2-30, M2T-32) with rms3, and one (M3T-946) with rms4. As for the previous study (15), no additional ramosus genes were identified. Including these new mutant alleles, a total of 11 mutant alleles are now known for the Rms1 locus, 2 for Rms2, 5 for Rms3, 5 for Rms4 and 3 for Rms5. In a mutagenesis program, the probability of screening a new gene is very low when 5 to 6 alleles are already known per locus. Consequently, the number of Rms genes in pea which give both profuse basal and aerial branching when mutated may be reaching saturation. The Rms1 gene seems more susceptible to mutation with 11 versus 2 to 5 mutants at the other loci. The rms1, rms3 and rms4 mutants have now been obtained in both tall and dwarf backgrounds whereas rms2 mutants have been selected only in tall backgrounds and rms5 in dwarf backgrounds. In order to map the rms2 gene, we crossed the tall mutant line K524 (rms2, Af, Le) with Terese (Rms2, af, le). In the F2 and F3 generations, plants with the phenotype rms2, af, le have been identified. This phenotype is quite different from that of the mutant lines obtained in the present work: the plants are much shorter and darker, with profuse branching from the first 3 or 4 nodes only. During our mutagenesis program, several mutants were obtained with decreased stature but only small increases in basal branching. These mutants were not selected as potential branching mutants in the screens described because some mutations that diminish internode length (e.g. le or na) also tend to cause increased basal branching (13). New allelism tests will have to be performed to determine if some of these induced mutant lines with decreased stature may contain a mutant rms2 allele.
The five new ramosus lines identified here have potential value for basic research. However, because of their profuse branching at aerial nodes, they have no perceived agronomic relevance. During this mutagenesis program, a screen using cytokinin applications at node 2 was performed to isolate mutants specifically altered in apical dominance at basal nodes. Such lines may have more agronomic value. However, the general aim of the mutagenesis program is to obtain a diverse range of mutants with altered branching patterns.
Acknowledgments. We thank Mr Mike Ambrose, John Innes Centre, for providing seed of WL5237 (JI1745), and the Union Nationale Interprofessionnelle des Plantes Riches en Protéines (UNIP) and the Australian Research Council for financial support.
______________________
1. Apisitwanich, S., Swiecicki, W. K. and Wolko, B. 1992. Pisum Genetics 24:14-15.
2. Arumingtyas, E. L., Floyd, R. S., Gregory, M. J. and Murfet, I. C. 1992. Pisum Genetics 24: 17-31.
3. Beveridge, C. A., Murfet, I. C., Kerhoas, L., Sotta, B., Miginiac, E. and Rameau, C. 1997. Plant J. 11:339-345.
4. Beveridge, C. A., Ross, J. J. and Murfet, I. C. 1994. Plant Physiol. 104:953-959.
5. Beveridge, C. A., Ross, J. J. and Murfet. I. C. 1996. Plant Physiol. 110:859-865.
6. Blixt, S. 1976. Agri Hort. Genet. 34:83-87.
7. Coïc, Y. and Lessaint, C. 1971. Hortic. Fr. 8: 11-14.
8. Doré, T. 1992. Thèse de Doctorat de 1’INA Paris-Grignon, Paris.
9. Hedley, C. L. and Ambrose, M.J. 1981. Advances in Agronomy 34:225-277.
10. Jeuffroy, M. H. 1991. Thèse de Doctorat, Univ. Paris-Sud, Centre d'Orsay.
11. Murfet, I. C. and Reid, J. B. 1974. Z. Planzenphysiol. 71: 323-331.
12. Murfet, I. C. and Reid, J. B. 1985. In The Pea Crop: a Basis for Improvement, Eds P.D. Hebblethwaite, M. C. Heath and T.C.K. Dawkins, Butterworths, London, pp. 67-80.
13. Murfet, I. C. and Reid, J. B. 1993. In Peas - Genetics, Molecular Biology and Biotechnology, Eds R. Casey and D. R. Davies, CAB International, Wallingford, U.K., pp. 165-216.
14. Simmonds, N. W. 1981. Principles of Crop Improvement. Longman Inc., New York, pp. 257.
15. Symons, G. M. and Murfet, I. C. 1997. Pisum Genetics 29:1-6.
16. Wang, T. L., Hadavizideh, A., Harwood, A., Welham, T. J., Harwood, W. A., Faulks, R. and Hedley, C. L. 1990. Plant Breeding 105:311-320.
* * * * *