Epicuticular wax phenotype of the wel mutation and its effect on pea aphid populations in the greenhouse and in the field
Eigenbrode, S.D., White, C. and
Rohde, M. Department of Plant,
Soil and
Entomological
Sciences
University
of Idaho
Moscow ID 83843-2339, USA
Simon, C. J. USDA/ARS Plant Introduction Station
Pullman
WA 99164-6402, USA
Several mutations that reduce the abundance and crystallization of epicuticular wax have been described in peas (6, 7, 8, 9). The mutation wel (‘wax eliminator’) identified by Marx (9) is unique among reported wax mutations in peas in that it reduces visible waxbloom on all plant surfaces and throughout plant development. However, the chemical and wax morphological phenotype of wel has not been reported. In this paper we provide a description of the wel phenotype. Although similar ‘glossy’ mutations in other crops are sometimes associated with resistance to insects (2, 4), this has not been examined in peas. Because wel produces apparently the strongest effect among wax mutations in peas, we tested wel for effects on the pea aphic, Acyrthosiphon pisum.
Description of the wel phenotype
Segregants from one of Marx’s lines, housed in the G.A. Marx Pea Genetic Stock Collection at the USDA Plant Introduction Station in Pullman WA, USA, were used to generate two near-isogenic lines, 406G (glossy, wel/wel) and 406N (normal-wax, Wel/Wel). Both lines are fixed for the mutation tl (tendrill less) which converts all tendrils to leaflets. Waxes were characterized on field-grown plants in 1996. The wax morphology of the two pea lines was examined with scanning electron microscopy of the upper and lower surfaces of the leaf and stipule, and the stems of both lines. Samples were frozen in liquid nitrogen and lyophilized. Fragments were coated with gold for 10 or 20 seconds at 10 mA and examined at 15 KV. Multiple fields were examined and the densities of wax crystals of different types determined. The wax chemistry and wax load of the two lines were determined by extracting upper and lower leaf surfaces with hexane and analysing with GC-MS. Extracts were derivatised with bis(trimethyl-sily1)-acetimide. For quantification, the derivatised lipids were injected along with a hexadecane internal standard on a Shimadzu GC-17A gas chromatograph equipped with a flame ionization detector. Response factors were determined from known standards (pentacosane, heptacosane, nonacosane, triacontane, and trimethyl-silyl esters and ethers of tetradecanoic acid, hexadecanoic acid, octadecanoic acid, hexacosanoic acid, docosanol, octacosanol, and triacontanol), or approximated for components for which standards were not obtained. Identification of the components was on the basis of retention times and on mass spectra of representative pea wax samples on a Hewlett Packard 5890 gas chromatograph equipped with a quadrapole mass selective detector. Relative composition of the waxes was calculated on a percent basis. Total amounts of all components provided an estimate of the wax load in µg/cm2 of leaf surface.

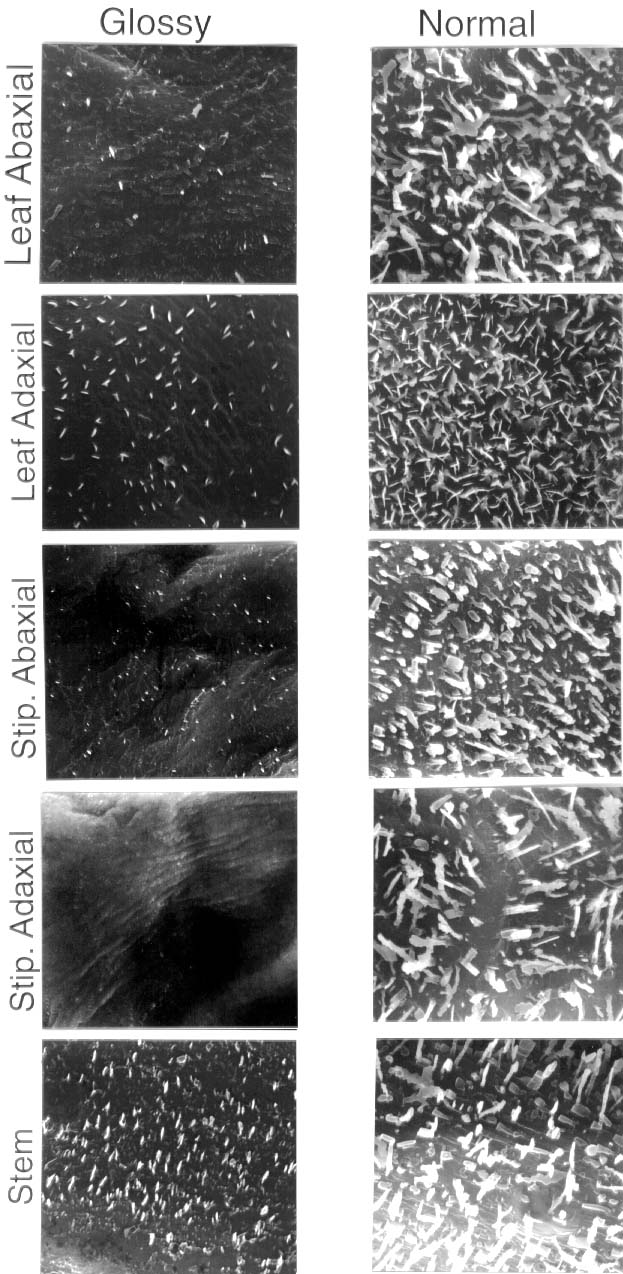
Fig. 1. Scanning electron micrographs of abaxial and adaxial surfaces of leaf and stipule (stip), and stem surfaces of 406G (wel/wel) and 406N (Wel/Wel) peas. The original width of each panel is 15 µm.
Density of typical prominent wax crystals was greatly reduced on all surfaces examined of 406G peas as compared with 406N peas (Fig. 1). Glossy waxes, especially on stems, retained relatively high densities of more minute crystals. For example, lower surfaces of 406G peas were devoid of crenate ribbons that occurred at densities of 55.1±10.5/100 µm2 on 406 N peas. However, the densities of all crystal types, including ? 1µm needles and granules were 103.7±14.2 and 90.7±74.7 per 100 µm2 (errors are standard deviations) on 406N and 406G, respectively.
Wax load (µg/cm2) on 406G was reduced to approximately one quarter and one seventh that on 406N on abaxial and adaxial leaf surfaces, respectively (Fig. 2 A). On abaxial leaf surfaces, the relative proportion of alkanes and secondary alcohols was decreased and primary alcohols, free fatty acids and wax esters was increased on 406G as compared with 406N (Fig. 2B). On adaxial leaf surfaces, the relative proportion of primary alcohols was decreased, and alkanes, secondary alcohols and free fatty acids was increased on 406G. The wel chemical phenotype is similar to other characterised wax mutations in Pisum in the enhanced proportion of wax esters on the abaxial leaf surface, and reduced alkanes on the abaxial surface (6). It is unique in the combination of effects on all constituent classes on different surfaces.
Insect tests
In the greenhouse, 406G and 406N pea plants with 12 expanded leaves ( the plants flowered at node 12) were infested with pea aphids, Acyrthosiphon pisum, from a virus-free colony maintained at the University of Idaho. In the first of the two experiments, initial aphid densities wree 10, 20, and 40 aphids per plant with 3 replications. In the second experiment, initial densities were 40 aphids per plant with 5 replications. Aphid densities at the end of the experiment (8 or 10 days) did not differ between the two lines in either experiment (Table 1). Lighting regime was the same in both experiments (h light, 8h dark), but warmer day temperatures in experiment 1 (26°day, 15° night) versus experiment 2 (22° day, 15°C night) caused slower overall aphid colony growth.
Natural populations of pea aphids and other insects were monitored in 1-m2 plots of 406G and 406N peas near Moscow, Idaho during summer of 1996 and 1997. There were 4 replications of 406G and 3 of 406N in 1996 and 4 replicates of each line in 1997. Aphids were monitored weekly until the crop dried down. Seasonal mean aphid densities were consistently and significantly lower on glossy 406G (P<0.001) plants than on the normal 406N plants (Fig. 3).
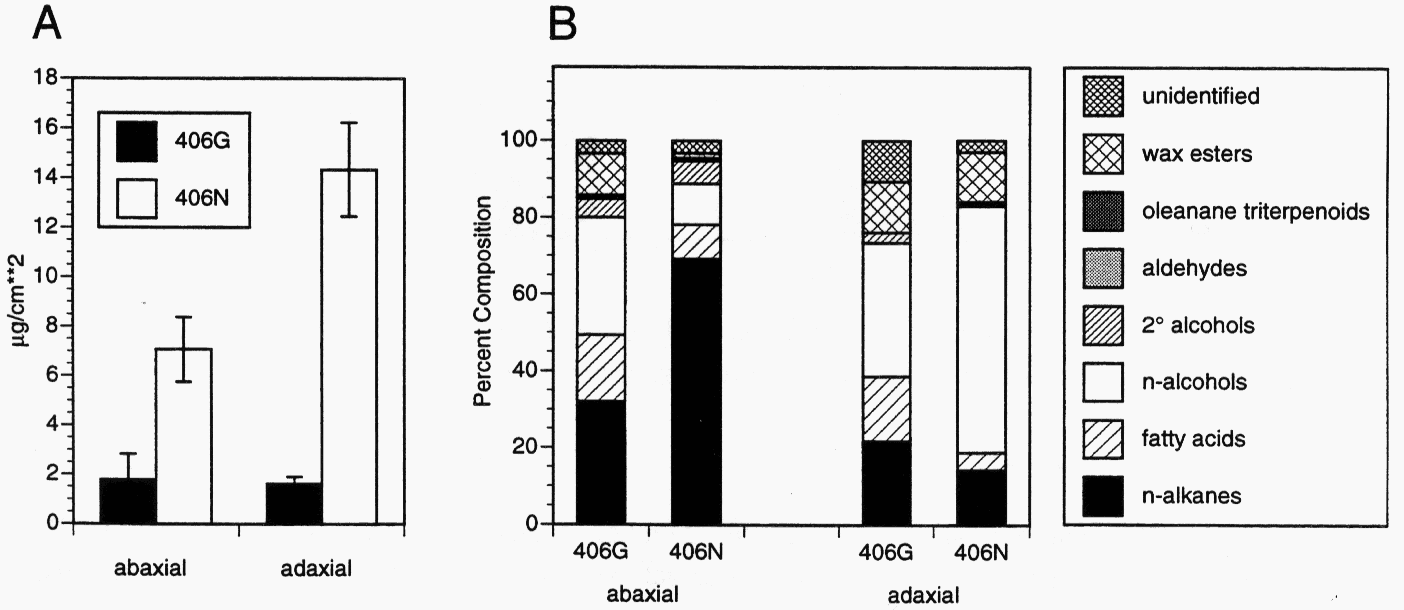
Fig. 2 A. The total amount of wax (µg/cm2) on leaf surface of 406G (glossy) and 406N (normal wax) peas. B. The percent composition by component class on leaf surfaces of 406G and 406N peas.
Table 1. Population growth of pea aphid on lines 406G (wel/wel) and 406N (Wel/Wel)
|
Final density |
(aphids per plant) |
||||
|
Experiment |
Starting density (aphids per plant) |
Days after infestation |
406G |
406N |
P value of t test |
|
10 |
8 |
354.0±86.8 |
260.7±27.7 |
0.36 |
|
|
20 |
8 |
433.3±66.9 |
369.7±67.8 |
0.54 |
|
|
40 |
8 |
681.0±116.7 |
730.3±174.8 |
0.83 |
|
|
40 |
10 |
244.8±41.2 |
296.6±46.5 |
0.83 |
|
Studies of the mechanisms of this resistance are underway. The dependence for expression of resistance on field conditions have prompted two lines of investigation: first, tests on the effect of water stress on aphid performance, following evidence that resistance to cabbage aphid in glossy Brassica is dependent on this factor (1), and second, tests on the effect of the wax bloom on predatory insects, following evidence that a reduced wax bloom on Brassica crops can enhance the mobility and efficacy of predators (3, 5).
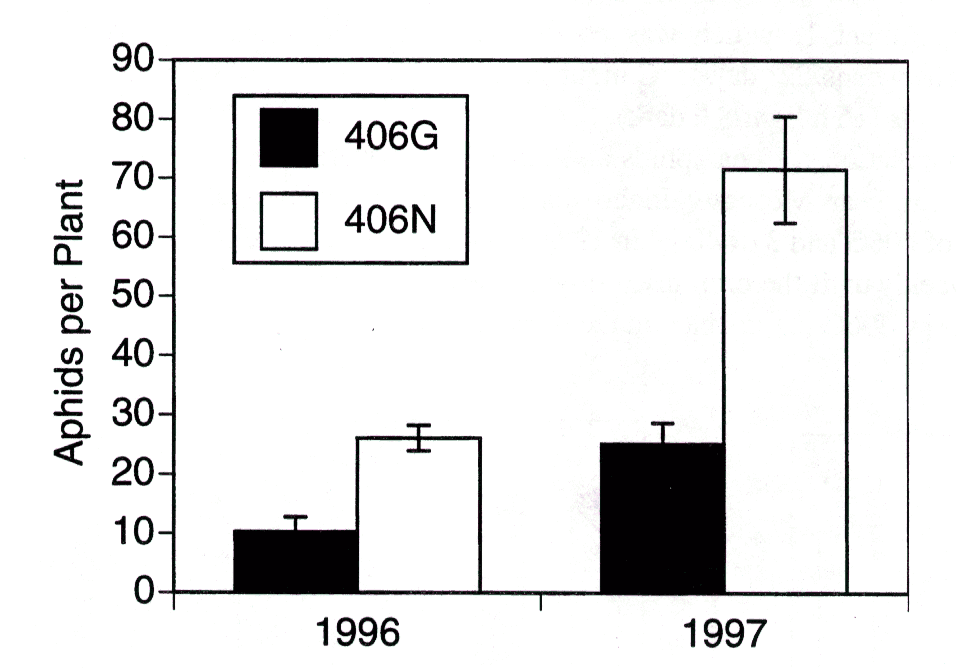
Fig. 3. Seasonal mean pea aphids per plant for lines 406N and 406G in the field. P values for t tests ? 0.001.
__________________
1. Cole, W.A. and Riggall, W. 1992. In: Proceedings on the 8th International Symposium of Insect-PlantRelationships. Pp. 313-315.
2. Eigenbrode, S.D. 1996. In: G. Kerstiens (ed), Bios Press, Oxford. Pp. 201-222.
3. Eigenbrode, S.D., Castagnola, T., Roux, M.-B. and Steljes, L. 1996. Entomologia Experimentalis et Applicata. 81:335-343.
4. Eigenbrode, S.D. and Espelie, K.E. 1995. Ann Rev Entomol. 40:171-194.
5. Eigenbrode, S.D., Moodie, S. and Castagnola, T. 1995. Entomologia Experimentalis et Applicata. 77:335-342.
6. Holloway, P.J., Hunt, G.M., Baker, E.A. and Macey, M.J.K. 1977. Chemistry and Physics of Lipids. 20:141-155.
7. Kovalenko, O.V. and Ezhova, T.A. 1992. Pisum Genetics 24:60-63.
8. Macey, M.J.K. and Barber, H.N. 1970. Phytochemistry. 9:5-12.
9. Marx, G.A. 1969. Pisum Newsletter. 1:10-11.
* * * * *