|
Pisum Genetics |
Volume 25 |
1993 |
Research Reports |
pages 53-59 |
Seed development in Pisum. Possible intragenic recombinations at the Lh locus
|
Swain, S.M. and Reid, J.B. |
Department of Plant Science, University of Tasmania Hobart , Tasmania 7001, Australia |
Two recessive alleles have been characterised at the Lh locus: lh and lhi Both of these alleles reduce GA1 (Gibberellin A1) levels in young shoots, thereby reducing internode elongation and causing a dwarf phenotype compared with wild-type (Lh) plants (4, 9). In shoots, the lh allele is more severe than lhi since lh plants possess lower GA1 levels in young shoots and are shorter than lhi plants (9). The lh and lhi alleles are also expressed in developing seeds. However, in developing seeds the lhi allele is more severe than the lh allele since lhi seeds possess lower GA levels than lh and wild-type seeds (10). Compared with wild-type and lh seeds, lhi seeds weigh less and are more likely to abort, suggesting that GAs play an important role in pea seed development (9, 10). Since the relative severity of the lh and lhi mutations varies between developing seeds and young shoots, the lh and lhi alleles demonstrate tissue-dependent regulation of GA-biosynthesis. This suggests that discrete regions of the Lh locus may be involved in the regulation of GA-biosynthesis in different tissues. Consequently, it may be possible to obtain additional mutant alleles at the Lh locus with tissue-dependent regulation of GA-biosynthesis different from that of the lh and lhi mutations. Such alleles may be of use in further investigating the physiological roles of the GAs. For example, if an additional allele was identified which reduced GA levels in developing seeds, reduced seed weight and increased seed abortion, this would lend further support for a physiological role for GAs in seed development. Although mutagenesis programs may produce further mutations at this locus, new alleles might also arise if natural recombination within the Lh locus occurred during meiosis in heterozygous lhlhi plants.
The F1 and F2 progeny resulting from a cross between lines K511 (lh) and NGB5843 (lhi) have been described elsewhere (9). Although the majority of the F2 plants resembled either the lh or lhi parent with regard to both internode length and seed development, two plants did not (data not shown). One plant possessed internode lengths similar to NGB5843 (lhi) but seed development similar to K511 (lh), while the other possessed internode lengths similar to K511 (lh) but seed development similar to NGB5843 (lhi). One possible explanation for these novel phenotypes is that they result from intragenic recombination at the Lh locus. In this paper, these two genotypes are examined.
Materials and Methods
The pure lines of Pisum sativum L. used here are held in the collection at Hobart, Australia. The dwarf lines NGB5843 (lhi) and K511 (lh) are derived from the wild-type tall cv. Torsdag (4, 8). All lines are homozygous dominant at the internode length loci Le, Ls, Lh, Na, Lk, Lka, Lkb, Lkc, Lkd, Lv, Lw, La and/or Cry, Lm and Sln unless otherwise indicated. Further details about the phenotypes and genotypes of these lines can be found in Reid and Ross (6). The origin of lines L237 (lhf) and L238 (lhs) is described in the results section.
All plants from which seeds were harvested were grown at a density of 2 per pot, except for the F1 parents used to obtain the data shown in Table 2, which were grown at a density of 1 per pot. GA levels in developing seeds harvested at contact point (the first day no liquid endosperm remained) were determined as described in Swain and Reid (9). To compare the effects of paclobutrazol on lines K511 (lh), NGB5843 (lhi), L237 (lhf) and L238 (lhs), 1 mg of paclobutrazol was applied to the dry, nicked seed (n≥5) in 2 ml of ethanol before planting. Control plants (n≥8) received 2 ml of ethanol only. These plants were grown in tote boxes (9).
All plants were initially grown in a heated glasshouse (5). At three weeks of age the F1 plants from which the data in Table 2 were obtained, were transferred to a controlled environment cabinet with day and night temperatures of 15°C and 10°C, respectively (9).
Results
The two novel F2 plants from cross K511 (lh) x NGB5843 (lhi) were allowed to self-pollinate over a further 5 generations, at which time they appeared to be pure-breeding (data not shown). The plants with internode lengths similar to NGB5843 (lhi) but seed development similar to K511 (lh) were assigned Hobart line number 237 (tentatively assigned symbol lhf, fertile). The plants with internode lengths similar to K511 (lh) but seed development similar to NGB5843 (lhi) were assigned Hobart line number 238 (tentatively assigned symbol lhs, sterile). Details of the internode lengths and seed development of these lines are shown in Table 1. Internode length between nodes 6 and 9 decreased in the order Lh (wild-type), L237 (lhf), NGB5843 (lhi), K511 (lh) and L238 (lhs) (P<0.05). In terms of seed development, L237 (lhf) plants possessed significantly more (P<0.05) seeds per pod than all other genotypes (Table 1). The number of seeds per pod developing on wild-type (Lh) and K511 (lh) plants was not significantly different (P=l), but both these genotypes possessed significantly more (P<0.05) seeds per pod than NGB5843 (lhi) and L238 (lhs) plants (Table 1). The number of seeds per pod developing on NGB5843 (lhi) and L238 (lhs) plants was not significantly different (P>0.5, Table 1).
Previous results obtained from allowing heterozygous Lhlhi F1 plants to self-pollinate demonstrated that the observed proportion of tall (Lh-) and dwarf (lhilhi) F2 progeny did not agree with the expected 3:1 ratio due to zygotic selection against the lhilhi seeds (9, 10). In addition, GA-deficient lhilhi seeds weigh less than Lh- seeds developing on maternal plants possessing either genotype lhilhi (10) or Lhlhi (8). Consequently, the development of homozygous lhs, lhf and lhi seeds was compared with Lh- or lh- seeds that developed on the same heterozygous maternal plant (Table 2).
Table 1. Phenotypes of cv. Torsdag (wild-type), K511 (lh), NGB5843 (lhi), L237 (lhf) and L238 (lhs) plants. Values represent the means of at least 11 plants ± SE. Photoperiod 18 h.
|
Line |
Genotypea |
Stem length between nodes 6 and 12 (cm) |
Fully developed seeds per pod |
|
Torsdag |
Lh |
45.9±0.9 |
3.00±0.20 |
|
L237 |
lhf |
18.0±0.3 |
3.71±0.19 |
|
K511 |
lh |
11.9±0.1 |
3.00±0.26 |
|
NGB5843 |
lhi |
16.9±0.3 |
2.02±0.26 |
|
L238 |
lhs |
11.0±0.1 |
1.88±0.13 |
a lhf and lhs are tentative alleles.
Table 2. Observed segregation for number of plants and dry seed weights of the F2 progeny (seeds) resulting from self-pollination of Lhlhs, Lhlhf and lhilhi F1 plants grown in day (18 h) and night (6 h) temperatures of 15°C and 10°C, respectively.
|
Parent genotype (F1) |
Observed F2 segregation |
||||||
|
Dominant |
Recessive |
||||||
|
Genotype |
No. |
Weight (mg) |
Genotype |
No. |
Weight (mg) |
||
|
Lhlhs |
Lh- |
105 |
297±3 |
lhslhs |
12a |
245±9b |
|
|
Lhlhf |
Lh- |
69 |
268±3 |
lhflhf |
15 |
282±5c |
|
|
lhlhi |
lh- |
92 |
293±3 |
lhilhi |
7a |
236±7b |
|
a Significant deficiency (P<0.001) of homozygous recessive progeny.
b Homozygous recessive seeds significantly lighter (P<0.01) than seeds with at least one dominant allele.
c Homozygous lhf seeds significantly heavier (P<0.05) than Lh- seeds.
Heterozygous Lhlhs (tall phenotype), Lhlhf (tall phenotype) and lhlhi (dwarf phenotype) plants were allowed to self-pollinate in day and night temperatures of 15°C and 10°C, respectively, since these conditions allow differences between the final weight of Lh- and lhilhi seeds to be detected (8, 10). The resulting F2 seeds were individually weighed and subsequently sown to determine their genotype. To distinguish between genotypes lh- and lhilhi (from lhlhi parents), 0.5 mg of paclobutrazol (in 2 ml of ethanol) was applied to the dry seeds before sowing since lhi plants are more sensitive to paclobutrazol than lh plants (9). Approximately three weeks later seed genotypes were determined by scoring the phenotypes of the resulting seedlings. Heterozygous Lhlhs and Lhlhf parents produced either tall (Lh-) or dwarf (lhslhs or lhflhf) seedlings. Heterozygous lhlhi parents produced either dwarf (lh-) or nana (lhilhi) plants because of the different response to paclobutrazol (9). Having established the genotypes of individual seeds, mean seed weights were calculated for the different seed genotypes.
The Lh/lhs and lh/lhi alleles did not segregate in agreement with the expected 3:1 ratio due to a deficiency of lhslhs and lhilhi F2 progeny, respectively (P<0.001, Table 2). The mean seed weight of lhslhs and lhilhi seeds was also less than that of Lh-and lh- seeds, respectively (P<0.01, Table 2). By contrast, the Lh (wild-type) and lhf alleles segregated in agreement with the expected 3:1 ratio (Table 2, P>0.10). Furthermore, homozygous lhf seeds did not weight less than Lh- seeds, and in fact were slightly heavier (P<0.05, Table 2).
To compare GA levels in seeds from the various lines at the same developmental stage, seeds were harvested at contact point (the first day no liquid endosperm remained). Seeds of genotype lhi were found to weigh less and possess dramatically reduced levels of GA20 compared with wild-type (Lh) seeds (Table 3), consistent with previous results (10). The lh allele was also found to reduce endogenous GA20 levels, compared with the wild-type (Lh) allele, although lh seeds possessed markedly more GA20 than lhi seeds (Table 3). Fertilization of lhi flowers with lh pollen (from K511 plants) produced seeds containing embryos of genotype lhlhi. This increased seed weight and endogenous GA20 and GA29 levels, compared with self-pollinated lhi plants, to levels comparable to self-pollinated lh plants (Table 3). This result suggests that the lh allele is completely dominant over the lhi allele in developing seeds, consistent with the segregation of the lh and lhi alleles (Table 2) and previous results (10).
Endogenous GA levels were also determined in seeds harvested at contact point from L237 (lhf) and L238 (lhs) plants. Lines K511 (lh) and L237 (lhf) possessed similar seed weights and similar GA20 and GA29 levels in developing seeds (Table 3). Lines NGB5843 (lhi) and L238 (lhs) also possessed similar seed weights and similar GA20 and GA29 levels in developing seeds (Table 3). Seed weights and endogenous GA20 and GA29 levels were reduced in NGB5843 (lhi) and L238 (lhs) seeds compared with K511 (lh) and L237 (lhf) seeds (Table 2).
Table 3. Fresh weights and GA levels in seeds (including testa) harvested at contact point (the first day no liquid endosperm remained) for various genotypes at the Lh locus. Heterozygous lhlhi seeds were produced by fertilizing lhi (NGB5843) plants with lh (K511) pollen. GA1 and GA3 are not present in seeds at this developmental stage (1).
|
Experiment |
Line |
Seed genotypea |
Average seed weight at harvest (mg) |
GA level (ng.(gFW-1)) |
|
|
GA20 |
GA29 |
||||
|
|
Torsdag (wild-type) |
LhLh |
227 |
775 |
-b |
|
1 |
NGB5843 |
lhilhi |
195 |
14 |
- |
|
|
K511 |
lhlh |
255 |
165 |
- |
|
|
K511 |
lhlh |
280 |
248 |
37 |
|
2 |
NGB5843 |
lhilhi |
180 |
7 |
1 |
|
|
5843xK511 pollen |
lhilhi |
260 |
218 |
31 |
|
|
L237 |
lhflhf |
235 |
298 |
44 |
|
3 |
K511 |
lhlh |
229 |
278 |
33 |
|
|
NGB5843 |
lhilhi |
207 |
7 |
2 |
|
|
L238 |
lhslhs |
201 |
7 |
3 |
a lhf and lhs are tentative alleles.
b not measured.
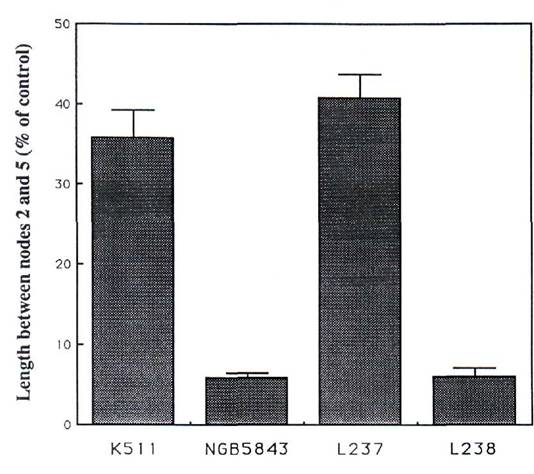
Since lhi plants are more sensitive to exogenously applied paclobutrazol than lh plants (9), the response of L237 (lhf) and L238 (lhs) plants to paclobutrazol applied to the dry seed before sowing was examined (Fig. 1). The degree of sensitivity to paclobutrazol was associated with seed GA levels and seed development rather than with the internode length of untreated plants. Compared with K511 (lh) and L237 (lhf) plants (relatively few aborting seeds), NGB5843 (lhi) and L238 (lhs) plants (relatively many aborting seeds) are extremely sensitive to applied paclobutrazol (P<0.001, Fig. 1).
Discussion
The levels of GA20 and GA29 in developing seeds from the four dwarf genotypes (Table 3) are consistent with an important role for the GAs in pea seed development (10) and they correlate with the mean number of seeds per pod observed for the four dwarf genotypes (Table 1) and with the development of homozygous recessive seeds on heterozygous parent plants (Table 2; 8). GA-deficient seeds (embryo genotypes lhilhi and lhslhs) are lighter and more likely to abort than seeds with higher GA levels (embryo genotypes Lh-, lh- and lhflhf).

Fig. 1. Response of lines K511 (lh), NGB5843 (lhi), L237 (lhf) and L238 (lhs) to 1 mg of paclobutrazol applied to the dry seed (in 2 ml of ethanol) before planting. Error bars represent SE's calculated on the basis of the stem length between nodes 2 and 5 for each of the treated plants relative to the mean for untreated controls of the same genotype.
The endogenous levels of GA1 in young shoots of L237 (lhf) and L238 (lhs) plants are also consistent with the observed internode length phenotypes of these lines (8). Taken together, the results for internode length, seed abortion, and endogenous GA levels in shoots and developing seeds of lines L237 (lhf) and L238 (lhs) are consistent with these lines having arisen from intragenic crossovers within the Lh locus. The response of the different dwarf genotypes to paclobutrazol (Fig. 1) suggests that the regions of the Lh locus responsible for (i) the increased sensitivity of lhi plants to paclobutrazol and (ii) GA levels in developing seeds co-segregated when both lines L237 (lhf) and L238 (lhs) arose. By contrast, the severity of the alleles in the shoot (i.e. GA1 levels) appears not to have segregated with seed GA levels or paclobutrazol response when these lines arose.
Intragenic crossovers have been reported in maize (e.g. Vp1 and R, 3, 7) and in Arabidopsis (e.g. GA1, 2). However, for the Lh locus, the relatively similar stature of the dwarf lines K511 (lh), NGB5843 (lhi), L237 (lhf) and L238 (lhs) does not allow the possible influence of another gene (also affecting intemode elongation) to be completely discounted. For example, lines K511 (lh) and NGB5843 (lhi) may also differ by an additional gene, with a relatively small effect on intemode elongation, that segregated in the F2 progeny of the cross between these lines. Consequently, the L237 and/or L238 phenotypes (Table 1) may not result from changes at the Lh locus. The segregation of intemode length phenotypes was examined in the F2 progeny resulting from crosses between L237 (Ihf) and cv. Torsdag (Lh), and between L238 (lhs) and cv. Torsdag (Lh), but the similar phenotypes of lines K511 (lh), NGB5843 (lhi), L237 (lhf) and L238 (lhs) prevented a clear interpretation of the results obtained (data not shown). Biochemical and/or molecular characterization of the Lh locus is required before the exact genetic nature of lines L237 (lhf) and L238 (lhs) can be determined.
Acknowledgements. We thank Katherine McPherson, Peter Bobbi and Peter Newman for technical assistance, and the Australian Research Council for financial support.
Gaskin, P., Gilmour, S.J.,
MacMillan, J. and Sponsel, V.M. 1985. Planta 163:
283-289.
Koornneef, M. 1979. Arabid. Inf. Serv. 16: 41-47.
McCarty, D.R. and Carson, C.B. 1991. Physiol. Plant. 81: 267-272.
Reid, J.B. 1986. Ann. Bot. 57: 577-592.
Reid, J.B. 1988. Physiol. Plant. 74: 83-88.
Reid, J.B. and Ross, J.J. 1993. Int. J. Plant Sci. 154: 22-34.
Robbins, T.P., Walker, E.L., Kermicle, J.L., Alleman, M. and Dellaporta, S.L.
1991. Genetics 129: 271-283.
Swain, S.M. 1993. Ph.D. Thesis (University of Tasmania).
Swain, S.M. and Reid, J.B. 1992. Physiol. Plant. 86: 124-130.
Swain, S.M., Reid, J.B. and Ross, J.J. 1993. Planta 191: 482-488.