|
Pisum Genetics
|
Volume 24
|
1992
|
Research Reports
|
pages 48-51
|
Twisted tendrils (Twt) -
a phenotype associated with a translocation involving chromosome 1
|
Gorel, F.L., Temnykh, S.V.,
Lebedeva, I.P., and
Berdnikov, V.A. |
Institute of Cytology and
Genetics
Novosibirsk 630090, Russia |
A new mutant displaying
altered tendril morphology was found in 1988 after treatment of Sprint-2 seeds
with 0.015% EMS. The tendrils of the mutant coil strongly soon after they form,
even in the absence of contact with other objects (Fig. 1). Three of four plants
in one M2 family expressed the mutant phenotype, and one of these was
used as the pollen parent in crosses with marker line WL1238 and with the
initial line Sprint-2. Approximately half of the progeny in both cases had
abnormal twisted tendrils and were semisterile; the other half were normal. We
initially hypothesised that the pollen parent was heterozygous for a dominant
gene that affects tendril morphology and fertility. The symbol Twt
(Twisted tendrils) is proposed.
An F2 population from
the cross WL1238 (A, twt, i, s, wb, k, b, le, gp, cp, tl, Ust,
Bra, fna) x Sprint-2 (a, Twt) was analysed, and a recombination
fraction of about 30% was found between genes Twt and a (data not
shown). For more accurate localisation of Twt a three-point test cross,
involving a, Twt, and His(2-6), was analysed. His(2-6) is a
cluster of closely linked genes coding a set of four molecular variants of
histone H1 (H1 haplotype) and has previously been shown to be 4 cM from a
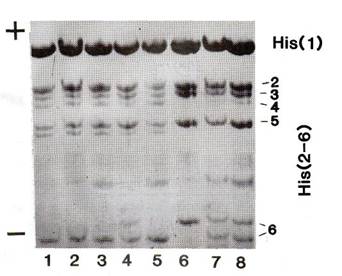
(1). The histone H1 phenotype is described by a numerical formula of four digits
that reflects the electrophoretic mobility of histone H1 variants numbers 3, 4,
5, and 6 (Fig. 2).
A maternal plant taken from VIR
accession K-3953 (Tadjikistan) with genotype H1-1133/H1-1133, A/A, twt/twt
was crossed with a plant having genotype H1-1021/H1-1021, a/a, Twt/twt
produced from the backcross mentioned above. Eight of the 15 F1
plants had phenotype Twt. The remaining seven plants were normal. This
result is consistent with the dominance of the mutant trait. All 8 F1
plants having supercoiled tendrils were crossed reciprocally with tester line
5-11 (H1-1123/H1-1123 a/a, twt/twt). Progeny analysis of
the testcross (Tables 1 and 2) permitted us to localise the genetic factor
responsible for phenotype Twt at 8 cM from a and 13 cM from
His(2-6). These data suggest the following order of the three genes:
His(2-6) -(5.3 cM) - a - (8.0 cM) - Twt.
However, it should be noted that
situation is complicated by the presence among the testcross progeny of 28
plants that apparently had all three sets of the histone H1 haplotype (see Fig.
2, lane 4). All of them had phenotype A, Twt, H1-1133/1021/1123,
and they could be distinguished from the rest by smaller size, much slower
development, and stipules with pointed apexes and undulating margins. These
observations, particularly the presence of all three H1 haplotypes, suggested
that these exceptional plants were trisomic at least with respect to the segment
of chromosome 1 carrying His(2-6). Indeed, the cytological analysis of
one of these trisomics revealed the presence of one additional chromosome (Fig.
3a).

|
Fig. 1. The phenotype of the
Twt (Twisted tendrils) mutant. |
|
Fig. 2. Segregation of histone
H1 phenotypes in testcross progeny. Lanes 1, 2, 3 and 5 show the
heterozygous phenotype 1133/1123 and lanes 7 and 8 show the
heterozygous phenotype 1021/1123. Lane 6 depicts the 1021 H1
pattern of Sprint-2. The trisomic phenotype 1021/1133/1123 is
represented by lane 4. |
|
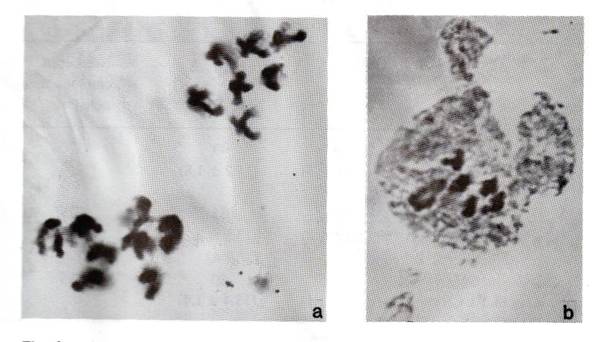
Fig. 3. Acetocarmine staining of
different meiotic stages. a) Eight and seven chromosomes at anaphase I in PMC of
a trisomic plant having phenotype A, Twt, H1-1133/1021/1123. b)
Five bivalents and one ring of four at metaphase I in PMC of a Twt/twt
plant from the cross K-3953 x Sprint-2 (Twt).
Table 1. Phenotypic
distribution in the progeny from the testcrosses:
1)
(Twt/twt, A/a, 1021/1133)
x (twt/twt, a/a, 1123/1123).
2)
(twt/twt, a/a, 1123/1123) x (Twt/twt, A/a, 1021/1133).
|
Phenotype |
|
|
|
Morphology |
H1 |
Testcross 1 |
Testcross 2 |
|
twt, A |
1133/1123 |
171 |
91 |
|
Twt, a |
1021/1123 |
150 |
107 |
|
twt, A |
1021/1123 |
13 |
9 |
|
Twt, a |
1133/1123 |
7 |
3 |
|
twt, a |
1021/1123 |
22 |
6 |
|
Twt, A |
1133/1123 |
7 |
13 |
|
Twt, A |
1133/1021/1123 |
21 |
7 |
Table 2. Total number and fraction
(in parentheses) of recombinant chromosomes in relation to gene pairs A,
His(2-6); A, Twt; and Twt, His(2-6).
|
Test-cross |
Gene pairs |
Number of chromosomes
tested |
|
A-His(2-6) |
A-Twt |
Twt-His(2-6) |
|
1 |
20 |
29 |
49 |
370 |
|
|
(5.4 ± 1.2) |
(7.8 ± 1.4) |
(13.2 ± 1.8) |
|
|
2 |
12 |
19 |
31 |
229 |
|
|
(5.2 ± 1.5) |
(8.3 ± 1.8) |
(13.5 ± 2.2) |
|
|
S
1+2 |
32 |
48 |
80 |
599 |
|
|
(5.3 ± 0.9) |
(8.0 ±1.1) |
(13.4 ± 1.4) |
|
The appearance of extra chromosomes
may be generated from lines heterozygous for a translocation (2). The trisomy we
observed in some of the Twt plants may have arisen as a result of a
translocation involving chromosome 1, with Twt residing at the point of
the chromosome break. This hypothesis is in agreement with two observations.
First, the pollen fertility of the heterozygous Twt/twt plants was
approximately 50-70% of that of the homozygous Twt/Twt and twt/twt
plants. Second, five bivalents and one ring of four chromosomes were seen at
metaphase 1 in pollen mother cells of heterozygous Twt/twt plants (Fig.
3b). The data suggest that EMS can induce structural aberrations in addition to
the point mutations. EMS is known to have produced deletions in maize (3). The
observations also could be interpreted to indicate that the mutant trait is
directly caused by the presence of reciprocal chromosome exchange. At present we
cannot determine if Twt is a phenotype produced by the translocation or
simply reflects an altered DNA sequence near the breakpoint. In either event,
Twt represents a unique case of an easily discernible dominant Mendelian
character associated with a translocation.
-
Belyaev, A.I. and Berdnikov, V.A.
1981. Genetika (USSR) 17:498-504.
-
Sutton, E. 1939. J. Genetics
38:459-476.
-
Okagaki, R.E., Neuffer, M.G. and
Wessler, S.R. 1991. Genetics 128:425-431.


