|
Pisum Genetics |
Volume 24 |
1992 |
Research Reports |
pages 40-43 |
Manifestation of the Lf locus in tissue culture of pea
|
Ezhova, T. A. and Kovalenko, O.V. |
Department of Genetics Moscow State University Moscow 119899, Russia |
Tissue and cell cultures provide excellent opportunities for studying the influence of nutrients on the morphological features of cultivated cells, the rate of cell division, and the regeneration capacity of plant cultures. The use, in in vitro experiments, of isogenic lines which differed for particular known genes allowed us to estimate the effect of these genes on the cultural characteristics mentioned above, and the dependence of this effect upon the composition of the culture media and conditions of cultivation. In this paper we report on the effect of genotype at the Lf locus and media composition.
Material and Methods
Earlier we obtained somaclonal variants from tissue culture of the pea cultivar Ranny Zeleny dealing with flowering behaviour (1). These somaclonal changes resulted from mutations at the Lf and Sn loci (7). Thus we had available for study the following isogenic lines which differed only in two genes:
LfLf snsn - initial cultivar Ranny Zeleny,
LfLf SnSn - line R1 (somaclonal variant),
lflf SnSn - line R9 (somaclonal variant),
lflf snsn - the line selected from the F2 population of cross R9 x Ranny Zeleny.
We studied the effect of exogenous growth regulators, NAA (napthalene acetic acid) and BA (benzyladenine), and genetic factors (dominant and recessive alleles of the Lf locus) on the growth of primary tissue cultures. The significance of data was tested by a Multifactor Analysis of Variance (Stratgraphics Statistical Graphics System, version 3 -computer program by Statistical Graphics Corporation, U.S.).
Tissue cultures were induced from shoot apices of 3-day-old sterile pea seedlings using the Gamborg method (2). All aspects of the experiment were carefully equalised, starting from the use of seeds of similar age, selection of seedlings with a similar degree of development, and ending with the use of explants of as near as possible equal weight The average fresh weight of explants was about 3-4 mg. Explants were cultivated on Gamborg et al media (2) in individual glass tubes (2 cm in diameter). Each treatment was replicated in 10-20 tubes. After randomization, the explants were cultivated at 26 ± 2°C in a 16 h photoperiod. Fresh weight was measured after 4 weeks.
Results and Discussion
In the preliminary experiment we found that the major factor which determines the difference in growth between tissues of genotype Lf and lf was the content of synthetic auxin in the culture media, not the cytokinin content. These results were confirmed in the following experiments.
Table 1. Analysis of variance for callus weight (experiment 1).
|
Source of variation |
d.f. |
Mean square |
F |
P |
|
Lf (Lf; lf) |
1 |
1251.61 |
0.920 |
0.349 |
|
NAA (0.2 mg/l; 1 mg/l) |
1 |
379.77 |
0.279 |
0.604 |
|
2-Factor interaction Lf-NAA |
1 |
11144.28 |
8.190 |
0.005 |
|
Residual |
116 |
1360.73 |
|
|
Table 2. Effect of NAA content of the medium on callus weight of genotypes Lf and lf (experiment 1).
|
Genotype |
NAA-content (mg/l) |
Count |
Mean ± SE (mg) |
|
Lf |
0.2 |
31 |
129.5 ± 5.6 |
|
|
1 |
28 |
152.7 ± 6,7 |
|
lf |
0.2 |
30 |
142.0 ± 7.9 |
|
|
1 |
31 |
126.6 ± 6.4 |
Table 3. Analysis of variance for callus weight (experiment 2).
|
Source of variation |
d.f. |
Mean square |
F |
P |
|
Lf(Lf, If) |
1 |
19618.19 |
7.998 |
0.006 |
|
BA (0.01; 0.1; 1 mg/l) |
2 |
380180.38 |
154.990 |
<0.001 |
|
2-Factor interaction Lf-BA |
2 |
2130.54 |
0.869 |
0.422 |
|
Residual |
114 |
2452.93 |
|
|
Table 4. Effect of B A content of the medium on callus weight of genotypes Lf and lf (experiment 2).
|
Genotype |
BA-content (mg/l) |
Count |
Mean ± SE (mg) |
|
Lf |
0.01 |
23 |
112.0± 6.5 |
|
|
0.1 |
11 |
161.5 ± 3.2 |
|
|
1 |
23 |
280.0 ± 14.3 |
|
lf |
0.01 |
21 |
133.1 ± 9.6 |
|
|
0.1 |
19 |
171.4 ± 11.0 |
|
|
1 |
23 |
319.9 ± 11.8 |
In the first experiment we tested the influence of two NAA concentrations on callus weight (0.2 and 1 mg/l NAA; 0.1 mg/l BA). The experiment showed that the major contribution to the variation in callus weight was made by the interaction between the factors Lf and NAA (P < 0.01, Table 1). These data indicated that alleles Lf and lf predispose the callus to respond differently to NAA. In the medium with a high auxin content, genotype Lf grew better than genotype lf whereas in the medium with a low auxin content genotype lf grew better than Lf (Table 2).
In the second experiment the influence of different BA content on callus weight was studied (0.01, 0.1 and 1 mg/l BA; 0,2 mg/l NAA). BA content of media was the main factor which contributed to the variation of callus weight (P < 0.001, Table 3). Callus growth increased with increasing concentration (Table 4). This can be explained by the fact that cytokinins stimulate cell division both in vivo and in vitro (5). No interaction between genotype at the Lf locus and BA content of the medium was observed. Thus genotypes Lf and lf responded similarly to the different content of cytokinin in culture media. A significant influence of the Lf locus on callus growth was observed (P < 0.01, Table 3) which is consistent with the results of the first experiment, where it was shown that genotype lf grew better than Lf in a medium with a low NAA content (0.2 mg/l in this case).
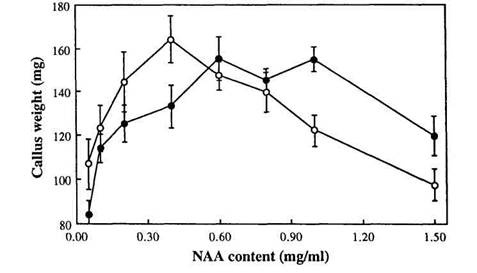
To study in more detail the effect of different NAA concentrations on the growth of genotypes Lf and lf, primary callus of lines R1 (Lf) and R9 (lf) was cultivated on media with a range of NAA concentrations from 0.05 to 1.5 mg/l (the concentration of BA was 0.1 mg/l in all 8 media variants). There was a significant interaction between Lf genotype and NAA concentration (F = 1.827, P < 0.01). Genotype lf grew better than Lf at concentrations of NAA from 0.05 to 0.4 mg/l whereas genotype Lf grew better than lf at concentrations of 1.0 to 1.5 mg/l (Fig. 1). At concentrations 0.6 and 0.8 mg/l there was no significant difference in growth between the two genotypes (Fig. 1).

Fig. 1. Mean callus weight plotted against NAA content of the medium for Lf (●) and lf (○) explants. SE bars are shown for all means; n = 7-17, mostly 8 or 9; data from experiment 3.
Thus expression of the Lf locus in tissue culture is manifest by an interaction between the Lf genotype and NAA concentration. Stimulation of callus growth by auxin can be connected with cell elongation as well as with activation of cell division (3, 8). However, an effect of a flowering gene on callus growth was unanticipated.
Among 9 genes which control flowering in pea, locus Lf occupies a special place because it determines the minimum node of flower initiation (6). Murfet (6) has proposed that allele Lf confers a higher threshold level for the flowering signal necessary to trigger flowering than allele lf. Thus the Lf locus may act in the apical meristem of plants to control the synthesis of a receptive protein, which interacts with the flowering signal and switches on the reproductive program of development. Our studies show that the Lf locus operates not only at the shoot apex but it also controls auxin responsiveness of callus cells in vitro. Similarly as in vivo, our data show that a higher threshold level of the growth regulator NAA is necessary to trigger active callus growth in genotype Lf than lf. These data can be interpreted as an indication that auxin plays the role of a flowering signal and that one of the auxin-binding proteins (ABP) is a product of, or controlled by, the Lf locus.
Jacobsen and Hajek (4) have shown the existence in pea of several ABPs with different characteristics and locations (membrane and cytoplasmic). The spectrum of ABPs may be distinct in different genotypes and in different tissues, and it can vary during ontogenesis (4). A study of the ABPs of isogenic lines of pea with different flowering genes would permit the correctness of our supposition regarding the action of Lf to be tested.
Acknowledgement. We thank the Russian Ministry of Science, High School and Technical Policy for financial support of our work.
Ezhova, T.A., Bagrova, A.M. and Gostimski, S.A. 1990. Pisum Newsl. 22:15-17.
Gamborg, O.L., Constabel, F., and Shyluk, J.P. 1974. Physiol. Plant. 30:125-128.
Gautheret, R.J. 1955. Ann. Rev. Plant Phys. 6:433-484.
Jacobsen, H.-J. and Hajek, K. 1987. In: Molecular Biology of Plant Growth Control, Alan R. Liss, Inc., pp. 257-266.
Miller, C.O., Skoog, F., von Saltza, M.H. and Strong, F.M. 1955. J. Am. Chem. Soc. 77:1392.
Murfet, I.C. 1985. In: Halevy, A.H., ed., Handbook of Flowering Vol. 4, CRC Press, Boca Raton, pp. 97-126.
Murfet, I.C. and Ezhova, T.A. 1991. Pisum Genetics 23:19-25.
Went, F.W. 1928. Rec. Trav. Bot. Neer. 25:1-116.