|
Pisum Genetics |
Volume 24 |
1992 |
Research Reports |
pages 17-31 |
Branching in Pisum: inheritance and allelism tests with 17 ramosus mutants
|
Arumingtyas, E.L., Floyd, R.S., Gregory, M.J. and Murfet, I.C.
|
Department of Plant Science University of Tasmania Hobart, Tasmania 7001, Australia |
Two genes, Fr and Fru, have been proposed by Lamprecht (9) and Blixt (3) to control the number of secondary stems in pea. Continuous unimodal F2 distributions for number of branches were partitioned arbitrarily into a 15:1 ratio (Fr Fru, Fr fru, fr Fru low: fr fru high) by Lamprecht and a 9:7 ratio (Fr Fru low: Fr fru, fr Fru ,fr fru high) by Blixt. Both authors pointed out branching is a difficult trait for Mendelian analysis since expression is very subject to environmental influences. Lamprecht considered the genes unsuitable for linkage analysis because of the high variability of expression, but Blixt (3) succeeded in placing Fr on chromosome 3 and Fru on chromosome 4.
Two ramosus (branching) mutants with clear expression have been induced in cv. Parvus. The ram mutant showed extreme proliferation of branches and a high number of flowers but was poorly fertile (11). Ram is located on chromosome 2 (10). Blixt (4) reported a second mutant, rms, which showed a 2.5-fold increase in number of basal branches compared with Parvus, plant height was reduced by 48%, and the stem was thinner but more woody and stiff. The rms locus is on chromosome 3 (4). Uzhintseva and Sidorova (27) obtained from cv. Torsdag a line, K319, with increased branching which appeared to result from a dominant mutation since the trait was expressed in the F1 hybrid between K319 and the initial line.
The flowering and internode length genes in pea have also been shown to exert a major influence over branching habit. In both pea and sweet pea, photoperiodic lines have a much greater tendency to produce basal laterals than lines with a day neutral flowering habit (5, 6, 20, 26) and this probably reflects the fact that they direct a greater flow of assimilate in a basipetal direction than day neutral types (2). The ability to respond to photoperiod is conferred in pea by the joint presence of dominant genes Sn and Dne (1, 8, 13) and activity of the Sn Dne system is reduced in long days (19). Hence long day conditions diminish the occurrence of basal laterals in photoperiodic genotypes, and outgrowth of these secondary stems may be completely suppressed in some circumstances (6). The flowering genes also influence the occurrence of aerial laterals which arise from the upper nodes. In pea, nodes which bear an inflorescence do not normally produce a lateral shoot. Hence genes such as Lf which delay the onset of flower initiation, increase the number of vegetative nodes potentially capable of producing laterals (6). In general, mutations which diminish internode length in pea by blocking gibberellin synthesis (e.g. le and na; 7, 21, 22) also increase the tendency to produce basal branches (6). In contrast, branching is generally reduced in short internode mutants where the elongation process is blocked some distance down the transduction pathway beyond the point of gibberellin reception, e.g. lk (23). The short internode double mutant K202 (ls lkc), which is blocked both prior to and after gibberellin reception (23, 24), shows a reduction in branching (6).
We report here on inheritance and allelism tests conducted over a period of several years on 17 induced ramosus mutants in pea.
Materials and Methods
Details of the 17 ramosus mutants are given in Table 1. Four of the initial lines are tall (Le): Kaliski (Wt4042), Parvus (L77), Torsdag (L107) and Weitor (WL1263) and four are dwarf (le): Meteor (L136), Paloma (Wt3527), Porta (Wt3519) and Raman (WL2168).
Table 1. Details of the pea ramosus mutants studied for inheritance and allelism.
|
Mutant linea |
Other names |
Initial lineb |
Mutagenic |
Author |
Supplied by |
|
K164 |
WL5847 |
Torsdag |
EMS |
K.K. Sidorova |
S. Blixt |
|
K319 |
L109 |
Torsdag |
NEU |
K.K. Sidorova |
K.K. Sidorova |
|
K487 |
WL5861 |
Torsdag |
NMU |
K.K. Sidorova |
S. Blixt |
|
K524 |
WL5864 |
Torsdag |
EMS |
K.K. Sidorova |
S. Blixt |
|
K564 |
WL5867 |
Torsdag |
EMS |
K.K. Sidorova |
S. Blixt |
|
K586 |
WL5868 |
Torsdag |
EMS |
K.K. Sidorova |
S. Blixt |
|
WL5147 |
– |
Weitor |
X-rays |
S.Blixt |
S. Blixt |
|
WL5237 |
– |
Parvus |
X-rays |
S.Blixt |
S. Blixt |
|
WL5918 |
II/77 |
Raman |
15 krad gamma |
M. Vassileva |
S. Blixt |
|
WL5951 |
L162 |
Parvus |
EMS 0.35% |
S. Blixt |
S. Blixt |
|
WL6042 |
IV/107 |
Meteor |
5 krad
g
+ |
M. Vassileva |
S. Blixt |
|
Wt10852 |
– |
Paloma |
0.014% NEU |
W.K. Swiecicki |
W.K. Swiecicki |
|
Wt15236 |
– |
Paloma |
– |
W.K. Swiecicki |
W.K. Swiecicki |
|
Wt15240 |
– |
Kaliski |
0.014% NEU |
W.K. Swiecicki |
W.K. Swiecicki |
|
Wt15241 |
– |
Paloma |
– |
W.K. Swiecicki |
W.K. Swiecicki |
|
Wt15242 |
– |
Paloma |
0.014% NEU |
W.K. Swiecicki |
W.K. Swiecicki |
|
Wt15244 |
– |
Porta |
170 rNf |
W.K. Swiecicki |
W.K. Swiecicki |
a Prefixes: K = Novosibirsk catalogue number, WL = Weibullsholm line number and Wt = Wiatrowo accession number.
b The initial cultivars were represented in this study by the following lines in parentheses: Kaliski (Wt4042), Meteor (Hobart line 136), Paloma (Wt3527), Parvus (Hobart line 77), Porta (Wt3519), Raman (WL2168), Torsdag (Hobart line 107) and Weitor (WL1263).
Table 2. Results for the F1, F2 and F3 of crosses between 14 pea branching mutants and their initial lines.
|
Cross |
F1 |
F2 segregation |
Chi-square testing 3:1 |
F3 from |
|
|
WTa |
Ma |
||||
|
K164 x Torsdag |
WT |
34 |
13 |
0.18 |
M |
|
K487 x Torsdag |
WT |
46 |
18 |
0.33 |
M |
|
K524 x Torsdag |
WT |
35 |
13 |
0.11 |
M |
|
K564 x Torsdag |
WT |
38 |
10 |
0.44 |
M |
|
WL5147xWeitor |
WT |
66 |
13 |
2.86 |
M |
|
WL5918 x Raman |
WT |
30 |
10 |
0.00 |
M |
|
WL5951 x Parvus |
WT |
34 |
14 |
0.44 |
M |
|
WL6042 x Ramanb |
WT |
30 |
18 |
4.00* |
M |
|
Wt10852 x Paloma |
WT |
42 |
11 |
0.51 |
M |
|
Wt15236 x Paloma |
WT |
36 |
8 |
1.09 |
M |
|
Wt15240 x Kaliski |
WT |
33 |
15 |
1.00 |
M |
|
Wt15241 x Paloma |
WT |
35 |
13 |
0.11 |
M |
|
Wt15242 x Paloma |
WT |
43 |
15 |
0.02 |
M |
|
Wt15244 x Porta |
WT |
35 |
7 |
1.56 |
M |
* P <0.05
a WT = wild type phenotype; M = mutant phenotype.
b Meteor is listed as the initial line but Raman was used because both WL6042 and Raman have an L-type flowering phenotype. The Meteor material in our collection has an early, day neutral flowering phenotype.
All mutant lines except K319, and all initial lines except Meteor, are late.flowering with a quantitative response to photoperiod (Murfet's L-type, 12) and are believed to have a flowering genotype of Lf Sn Dne hr (see 8, 14, 17, 18). K319 is a double mutant and is early flowering as a result of mutation of Lf to lf (15, 27, 28). The cv. Meteor in our possession is an early flowering, day neutral type (presumed to be lf sn Dne hr) and it therefore has a different flowering phenotype to mutant WL6042. Apart from the difference in branching habit, mutant WL6042 matched cv. Raman fairly closely in phenotype and the cross Raman x WL6042 was therefore used to check the inheritance of this mutant. All other inheritance tests were made by crossing the mutant with the listed initial line (Table 2). All such crosses were grown to F3. In total, 128 reciprocal crosses were made to test for allelism (Table 3).
The study was conducted in our glasshouse and controlled environment facilities. Plants were grown, one per pot, in 14 cm slimline pots filled with a 1:1 (v:v) mixture of vermiculite and 10 mm dolerite chips topped with 3-4 cm of sterilized peat-sand potting mixture. Liquid nutrient was supplied once per week in the form of Hoaglands #1 solution, Aquasol, or Total Growth Nutrient (R&D Aquaponics, Sydney).
Table 3. Diallel table showing the results (A=allelic, NA=not allelic) of crosses among 17 ramosus mutant lines of peas.
|
|
WL |
WL |
WL |
WL |
K164 |
K319 |
K487 |
K524 |
K564 |
K586 |
Wt |
Wt |
Wt |
Wt |
Wt |
Wt |
|
WL5918 |
A |
_ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
WL5951 |
NA |
NA |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
WL6042 |
|
NA |
NA |
_ |
|
|
|
|
|
|
|
|
|
|
|
|
|
K164 |
NA |
NA |
NA |
NA |
_ |
|
|
|
|
|
|
|
|
|
|
|
|
K319 |
NA |
NA |
NA |
NA |
NA |
_ |
|
|
|
|
|
|
|
|
|
|
|
K487 |
NA |
NA |
NA |
A |
NA |
NA |
_ |
|
|
|
|
|
|
|
|
|
|
K524 |
NA |
NA |
A |
NA |
NA |
NA |
NA |
_ |
|
|
|
|
|
|
|
|
|
K564 |
NA |
NA |
NA |
A |
NA |
NA |
A |
NA |
_ |
|
|
|
|
|
|
|
|
K586 |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
_ |
|
|
|
|
|
|
|
Wt10852 |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
_ |
|
|
|
|
|
|
Wt15236 |
A |
|
|
NA |
|
|
NA |
|
|
|
NA |
_ |
|
|
|
|
|
Wt15240 |
A |
A |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
A |
_ |
|
|
|
|
Wt15241 |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
A |
NA |
NA |
_ |
|
|
|
Wt15242 |
NA |
NA |
NA |
NA |
A |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
_ |
|
|
Wt15244 |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
A |
NA |
NA |
A |
NA |
_ |
|
WL5147 |
A |
A |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
A |
A |
NA |
NA |
NA |
Several photoperiods (24, 18, 16, 15, 12 and 8 h) were used in various stages of the study in order to characterise the mutants and to establish the conditions required to maximise separation of normal and mutant types while minimising the space and time necessary for this large scale program. Most of the tests were conducted under an 18 h photoperiod comprising natural daylight extended before dawn and after dusk by light from a mixed fluorescent and incandescent source (25 mmol m–2 s–1 at pot top). However, line K319 did not branch under these conditions and all tests involving that line were conducted under an 8 h photoperiod (8 h daylight + 16 h dark). Light quality and temperature varied with the seasons but temperature was generally controlled within the range of 20-25°C by day and 14-18°C at night.
Several variables were recorded including the node of origin of lateral branches and their length, the number of expanded leaves on each lateral, and the height of the main stem. In crosses involving parents of the same length genotype, total lateral length (TLL = sum of the lengths of all laterals present) generally proved a sufficient variable for distinguishing normal and mutant types. However, since the ramosus mutants often had a shorter main stem than the initial line, the ratio of TLL to main stem height (TL = total length of main stem) proved a more efficient discriminative variable, and essential in crosses between tall and dwarf types to diminish the confounding effect of the Le-le difference.

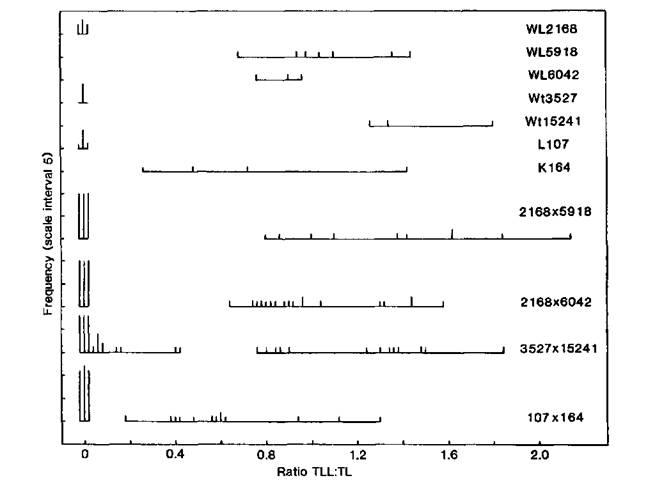
Fig. 1. Distribution of the ratio TLL (total lateral length): TL (total length of main stem) for initial lines Raman (WL2168), Paloma (Wt3527) and Torsdag (L107), ramosus mutants WL5918, WL6042, Wt15241 and K164, and the F2 of crosses between the mutants and their initial lines. Photoperiod 18 h. All data were recorded from 34-day-old plants except for Wt3527, Wt15241 and F2 where mature plants were measured.
Results and Discussion
Fifteen mutants proved readily amenable to Mendelian analysis. These 15 mutants all branched extensively under an 18 h photoperiod while their initial lines showed little or no branching. The F1 hybrids between the mutants and their initial lines were normal and the F2 segregated clearly in most cases. Examples are given in Figs 1 and 2. The observed F2 numbers were generally in good agreement with a 3 normal: 1 mutant ratio (Table 2), although cross WL6042 x Raman contained a slight excess (P < 0.05) of mutant types. Mutant-type F2 segregates bred true in F3. In all, there is little reason to doubt that these 15 mutants show single gene, recessive inheritance. Allelism tests showed these 15 mutants belonged to five loci (Table 3). Mutants WL5147, WL5918, Wt15236 and Wt15240 were allelic with mutant WL5237, the type line for rms (= rms-1). The remaining 10 mutants were assigned to four new ramosus loci designated rms-2, rms-3, rms-4 and rms-5 (Table 4).
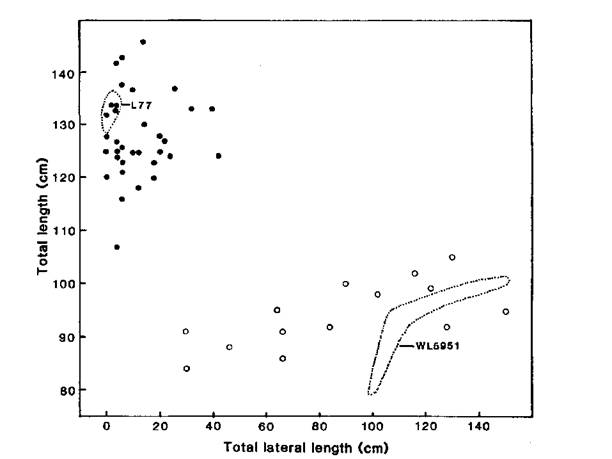
Fig. 2. Total length of the main stem (TL) plotted against total lateral length (TLL) for mutant WL5951 and its initial line Parvus (L77), and the F2 (●, ○) of cross WL5951 x Parvus. Photoperiod 18 h.
Table 4. Allelic series for 17 pea ramosus mutants.
|
Series |
Line |
Locus |
|
1 |
WL5147, WL5237*, WL5918, Wt15236, Wt15240 |
rms-1 |
|
2 |
WL5951, K524* |
rms-2 |
|
3 |
WL6042, K487*, K564 |
rms-3 |
|
4 |
K164*, Wt15242 |
rms-4 |
|
5 |
Wt10852, Wt15241, Wt15244* |
rms-5 |
|
6 |
K586 |
|
|
7 |
K319 |
|
*Type lines for rms-1 [Blixt (4)], rms-2, rms-3, rms-4 and rms-5 (the type line for rms-5 is designated in agreement with Apisitwanich et al, this issue pp. 12-13).
Examples of F2 segregation for each of the five rms loci are given in Figs 1 and 2. Segregations for rms-1 (Raman x WL5918), rms-3 (Raman x WL6042) and rms-4 (Torsdag x K164) each showed a qualitative separation into non-branching and branching types (Fig. 1). Segregation for rms-5 (Paloma x Wt15241) resulted in a distinct separation into normal and mutant types but in this case the difference was a quantitative one between little or no branching and extensive branching (Fig. 1). F3 data confirmed that the two Paloma x Wt15241 F2 plants with TLL:TL ratios around 0.4 were genetically Rms-5/-. The data for rms-2 (Fig. 2) provide an example where neither of the two variables total lateral length (TLL) or the ratio TLL:TL allows a clear separation into normal and mutant types. In fact the distributions overlap in each case. However, a two-way plot of TL against TLL has permitted a clear separation. It may be seen that the rms-2 allele has reduced TL (plant height) by around 25% and a similar effect was noted in the other cross (Torsdag x K524) segregating for rms-2. Whether a cross gave a qualitative separation or a quantitative difference seemed to be more a function of the initial line and its genetic background rather than which rms locus was involved. In general, crosses involving Torsdag, Raman, Weitor or Kaliski gave qualitative segregations while crosses involving Parvus, Paloma or Porta produced at least some non-mutant segregates with some outgrowth of branches.
The situation in regard to the remaining two mutants, K319 and K586, remains unclear. Our results gave no indication of allelism with each other, or with any of the other 15 mutants tested. However, we could not obtain clear proof of monogenic inheritance for either K319 or K586 and have therefore not assigned gene symbols at this stage. Increased branching did not always express in K586 under an 18 h photoperiod. F2 data for cross Torsdag x K586 obtained under a 14 h photoperiod were consistent with the hypothesis of monogenic partially recessive inheritance of the mutant trait, but further tests are necessary to confirm that interpretation. Line K319 carries two mutations - one causing increased branching and the other early flowering (27). Hence in the cross Torsdag x K319 expression of branching is confounded by the occurrence of early flowering segregates which have fewer nodes available for the production of aerial laterals. However, even when branching was examined solely in the late-flowering F2 segregates under an 8 h photoperiod, we could obtain no clear evidence of single gene segregation. Thus we can draw no firm conclusions on the genetic basis for increased branching in K319.
In broad terms, five patterns of branching could be discerned among the mutants and their initial lines: complete absence of branches (N = no branches), branches from all, or almost all, vegetative nodes (C = complete), branches from the upper nodes only (A = aerial), branches from the basal nodes only (B = basal), and branches from both the upper and lower nodes separated by a region devoid of branches (G = gap). These five patterns are illustrated in Figs 3-7. The N pattern is shown by initial lines Raman (Fig. 3) and Torsdag (Fig. 5) in a 24 h photoperiod. The C pattern is shown by mutants WL5237 (rms-1, Fig. 4) and K164 (rms-4 16 h, Fig. 6). An A pattern is seen in initial lines Parvus (Fig. 4) and Torsdag (Fig. 5) in a photoperiod of 16-18 h. The B pattern is evident in several examples in Figs 3 and 7. In plants displaying a G pattern the gap often occurred in the general region of nodes 4 to 8, e.g. Parvus (Fig. 4) and Torsdag (Fig. 5) in 8 h, K524 (rms-2, Fig. 5), and K487 (rms-3, Fig. 6) in 16 h or 24 h conditions, but in some cases under short day conditions a gap occurred much higher up the plant, e.g. K487 in an 8 h photoperiod (Fig. 6).
All the mutants were characterised by increased branching. Branching pattern varied with photoperiod and also among mutants at the same locus depending on genetic background of the initial line, particularly in regard to the le locus. With the possible exception of rms-2 (Fig. 5), there was no indication that mutation at any particular locus resulted in a unique or distinct phenotype. Nevertheless, some differences in expression of rms-2, rms-3 and rms-4 are apparent (Figs 5 and 6) from a comparison of the branching patterns of the mutants K524, K487 and K164 which are all derived from Torsdag. The mutants Wt15236 (rms-1), Wt15242 (rms-4), Wt10852 (rms-5) and Wt15241 (rms-5) also represent a three-locus-series from one initial line (Paloma). In this case the rms-1 and rms-4 mutants displayed a similar C branching pattern under an 18 h photoperiod while the two rms-5 mutants varied in expression from C to G for Wt10852 and G to B for Wt15241 under these conditions.
The stimulation of basal lateral outgrowth which occurs in photoperiodic (Sn Dne) lines under short day conditions (5, 6, 20) is clearly evident in both the initial lines and the mutants (Figs 3-7). The decrease in photoperiod from 24, through 16, to 8 h resulted in a dramatic change in pattern in some cases. For example, in Raman (Fig. 3) the branching pattern changed from N in 24 h to B in 16 h and 8 h while in K164 (rms-4, Fig. 6) the pattern changed from A in 24 h, to C in 16 h, to G in 8 h and the strong growth of the basal laterals is apparent in both lines under 8 h conditions where these laterals match or exceed the length of the primary stem. Tall (Le) and dwarf (le) lines occurred among the mutants for rms-1, rms-3 and rms-4. For each locus it was clear that the tendency to produce basal laterals was stronger in le than Le lines and this may be seen in the patterns for the two rms-1 lines WL5918 (dwarf, Fig. 3) and WL5237 (tall, Fig. 4). Thus the effects of le and the Sn Dne system observed in normal lines (6) were still apparent in the ramosus mutants.
The ram mutant was thought to be extinct but we have recently obtained some seed of Monti's original mutant line P745d. The ram mutant has a distinct phenotype which is clearly different from that of any of the 17 ramosus mutants in the current study.
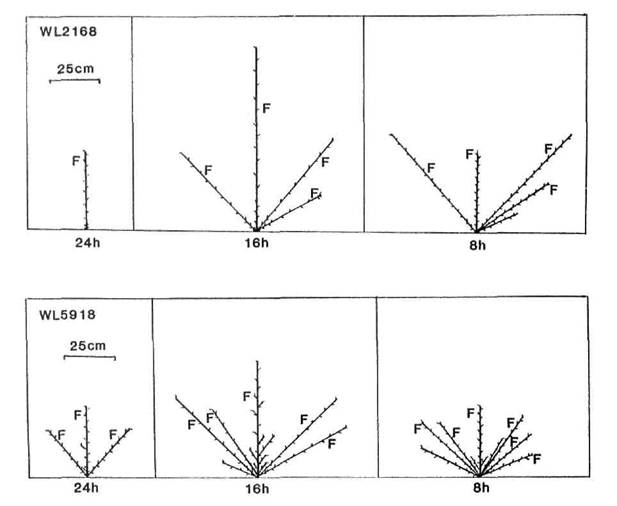
Fig. 3. Diagrammatic representation of mutant WL5918 (rms-1) and its initial line Raman (WL2168) grown under a 24, 16 or 8 h photoperiod. Shoot lengths are drawn to scale but not individual internodes. Only first order laterals are shown. F indicates the node of flower initiation. Raman is dwarf (le).
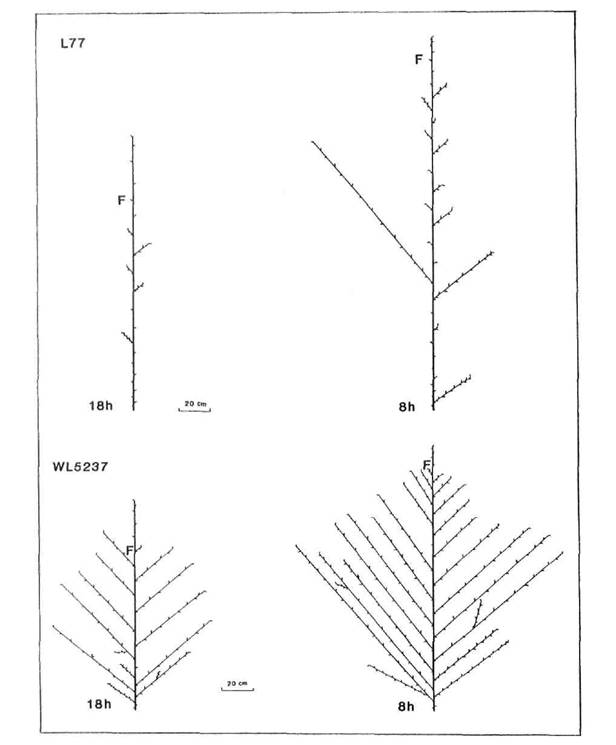
Fig. 4. Diagrammatic representation of mutant WL5237 (rms-1) and its initial line Parvus (L77) grown under an 18 or 8 h photoperiod. Shoot lengths are drawn to scale but not individual internodes. F indicates the node of flower initiation on the main shoot. Parvus is tall (Le).
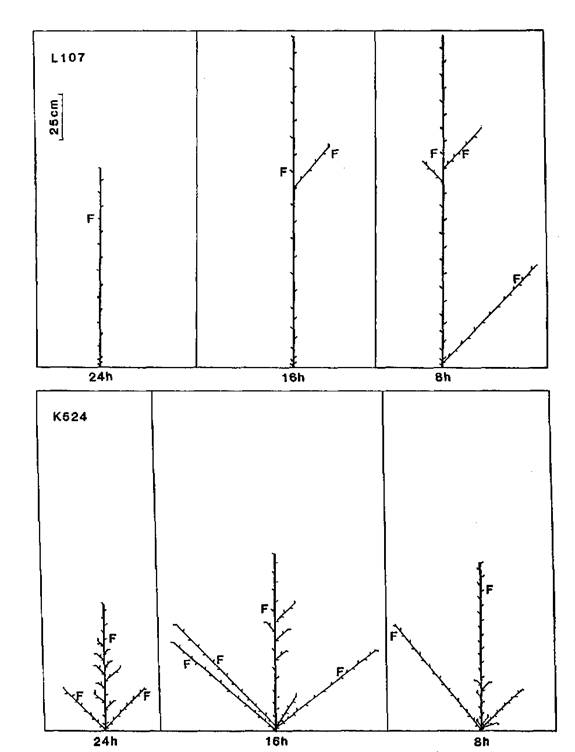
Fig. 5. Diagrammatic representation of mutant K524 (rms-2) and its initial line Torsdag (L107) grown under a 24, 16 or 8 h photoperiod. Shoot lengths are drawn to scale but not individual intemodes. Only first order laterals are shown. F indicates the node of flower initiation. Torsdag is tall (Le).
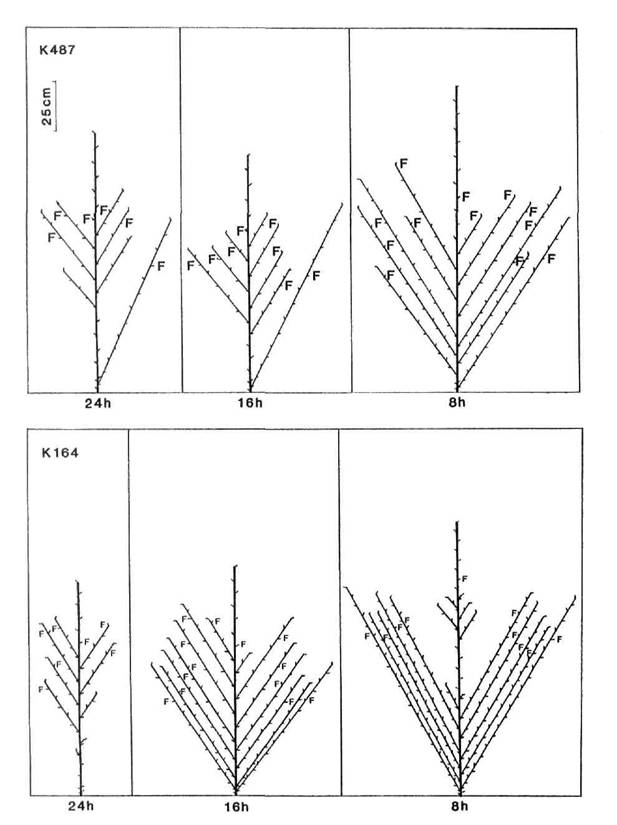
Fig. 6. Diagrammatic representation of mutants K487 (rms-3) and K164 (rms-4) grown under a 24, 16 or 8 photoperiod. Shoot lengths are drawn to scale but not individual internodes. Only first order laterals are shown. F indicates the node of flower initiation. The initial line Torsdag (L107) is tall (Le).
Plants homozygous for ram appear to suffer a progressive decline in apical meristem function and normal stem and leaf organogenesis, culminating in the failure of continued shoot growth. All 17 mutants in this study appear to possess normal meristem activity. As previously reported (11), the ram plants were poorly fertile. We conclude that gene Ram acts in a very different manner to the genes in the Rms series, and that it is extremely unlikely that Ram is allelic with any of the five Rms genes. It is already known that ram and rms-1 are located on different chromosomes (4, 10).
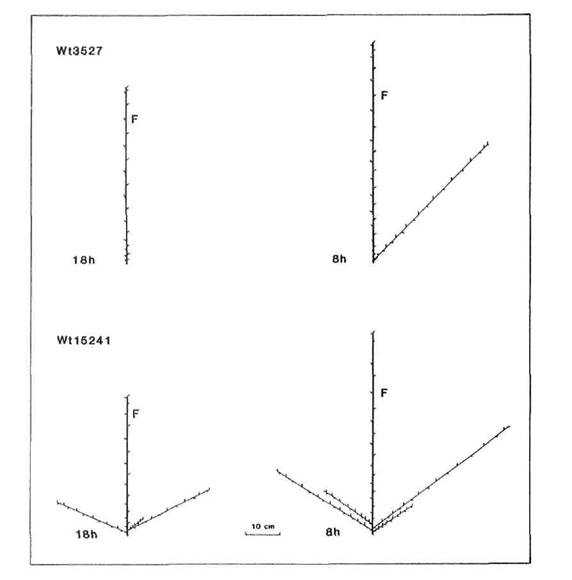
Fig. 7. Diagrammatic representation of mutant Wtl5241 (rms-1) and its initial line Paloma (Wt3527) grown under a 18 or 8 h photoperiod. Shoot lengths are drawn to scale but not individual intemodes. F indicates the node of flower initiation on the main stem. Paloma is dwarf (le).
Fifteen of the 17 mutants tested here showed clear monohybrid segregation with distinct expression of the mutant type. In contrast, the branching genes Fr and Fru have been proposed on the basis of arbitrary cuts in a continuous, unimodal distribution for number of stem branches (3, 9). No clear segregations for Fr-fr or Fru-fru have been reported. We felt it was not appropriate to include such weakly expressed material in our initial genetic analysis of the branching mutants. Nevertheless, Blixt's linkage data provide a strong indication that a certain region on each of chromosomes 3 and 4 is associated with the control of branching habit. It is of interest that Fr and Rms-1 both map to the same region of chromosome 3 (3, 4). Blixt reports a recombination fraction of 38% for St-Fr (3) and 41% for St-Rms-1 (4). The possibility is therefore raised that Fr and Rms-1 are the same gene. The flowering gene Hr also maps to this region of chromosome 3 with a recombination fraction of 41% for St-Hr (16). Hr also influences branching (25). Moreover, Blixt's cross Weitor x WL851 would be segregating for Hr-hr since our observations indicate Weitor has flowering genotype Sn Dne hr and WL851 genotype Sn Dne Hr. However, Hr promotes basal branching while Fr decreases basal branching, so segregation for Hr-hr does not explain Blixt's (3) data.
In conclusion, 15 branching mutants with clear expression have been shown to be under single gene recessive control and assigned to five ramosus loci, Rms-1 (4) and four novel loci Rms-2, Rms-3, Rms-4 and Rms-5. Further work is necessary to establish (a) the mode of inheritance of two additional branching mutants, K319 and K586, (b) the linkage relationships of Rms-2, Rms-3, Rms-4 and Rms-5, and (c) the relationship between Rms-1 and Fr. [Apisitwanich et al, this issue pp. 14-15, have shown the Rms-5 locus is on chromosome 5]. Mutants at these five Rms loci provide a valuable resource with which to explore the developmental control of branching and apical dominance in pea, and studies are currently underway on the biochemical and physiological action of the Rms genes.
Acknowledgements. We thank Dr S. Blixt, Dr K.K. Sidorova and Prof Dr W.K. Swiecicki for providing seed of the mutants, Mike Ambrose for locating material of ram, Peter Bobbi and Katherine McPherson for technical assistance, and the Australian Research Council for financial support.
Barber, H.N. 1959. Heredity 13:33-60.
Beveridge, C.A., Ross, J.J. and Murfet, I.C. 1992. J. Exp. Bot. 43:55-62.
Blixt, S. 1968. Agri Hort. Genet. 26:136-148.
Blixt, S. 1976. Agri Hort. Genet. 34:83-87.
Doroshenko, A.V. and Rasumov, V.I. 1929. Trudy prikl. Bot. Genet. Selek. 22:219-276.
Floyd, R.S. and Murfet, I.C. 1986. PNL 18:12-15.
Ingram, T.J., Reid, J.B., Potts, W.C. and Murfet, I.C. 1983. Physiol. Plant. 59:607-616.
King, W.M. and Murfet, I.C. 1985. Ann. Bot. 56:835-846.
Lamprecht, H. 1950. Agri Hort. Genet. 8:1-6.
Monti, L.M. 1970. PNL 2:21-22.
Monti, L.M. and Scarascia Mugnozza, G.T. 1967. Genetica Agraria 21:301-312.
Murfet, I.C. 1971. Heredity 26:243-257.
Murfet, I.C. 1971. Heredity 27:93-110.
Murfet, I.C. 1978. PNL 10:48-52.
Murfet, I.C. 1982. In Documentation of Genetic Resources: A Model, Eds S. Blixt and J.T. Williams, IBPGR, Rome, pp 45-51.
Murfet, I.C. 1988. PNL 20:29
Murfet, I.C. 1991. PNL 23:16-18.
Murfet, I.C. and Groom, K. 1984. PNL 16:57-58.
Murfet, I.C. and Reid, J.B. 1974. Z. Pflanzenphysiol. 71:323-331.
Murfet, I.C. and Reid, J.B. 1985. In The Pea Crop: a Basis for Improvement, Eds P.D. Hebblethwaite, M.C. Heath and T.C.K. Dawkins, Butterworths, London, pp 67-80.
Potts, W.C. and Reid, J.B. 1983. Physiol. Plant. 57:448-454.
Potts, W.C, Reid, J.B. and Murfet, I.C. 1982. Physiol. Plant. 55:323-328.
Reid, J.B. 1986. Ann. Bot. 57:577-592.
Reid, J.B., Ross, J.J. and Hasan, O. 1991. J. Plant Growth Regul. 10:11-16.
Ross, J.J. 1983. Ph.D. thesis, Univ. of Tas., Hobart.
Ross, J.J. and Murfet, I.C. 1985. Ann. Bot. 55:715-726.
Uzhintseva, L.P. and Sidorova, K.K. 1979. Genetika 15:1076-1082.
Uzhintseva, L.P. and Sidorova, K.K. 1988. PNL 20:39-40.