PNL Volume 19 1987 RESEARCH REPORTS
53
Cryptodwarf (la
cryc) types and particularly the more pronounced
slender (la crys) types have previously been shown to possess
reduced responses to treatments (either chemical or genetic) which
alter the level of active gibberellin (4,7).
slender (la crys) types have previously been shown to possess
reduced responses to treatments (either chemical or genetic) which
alter the level of active gibberellin (4,7).
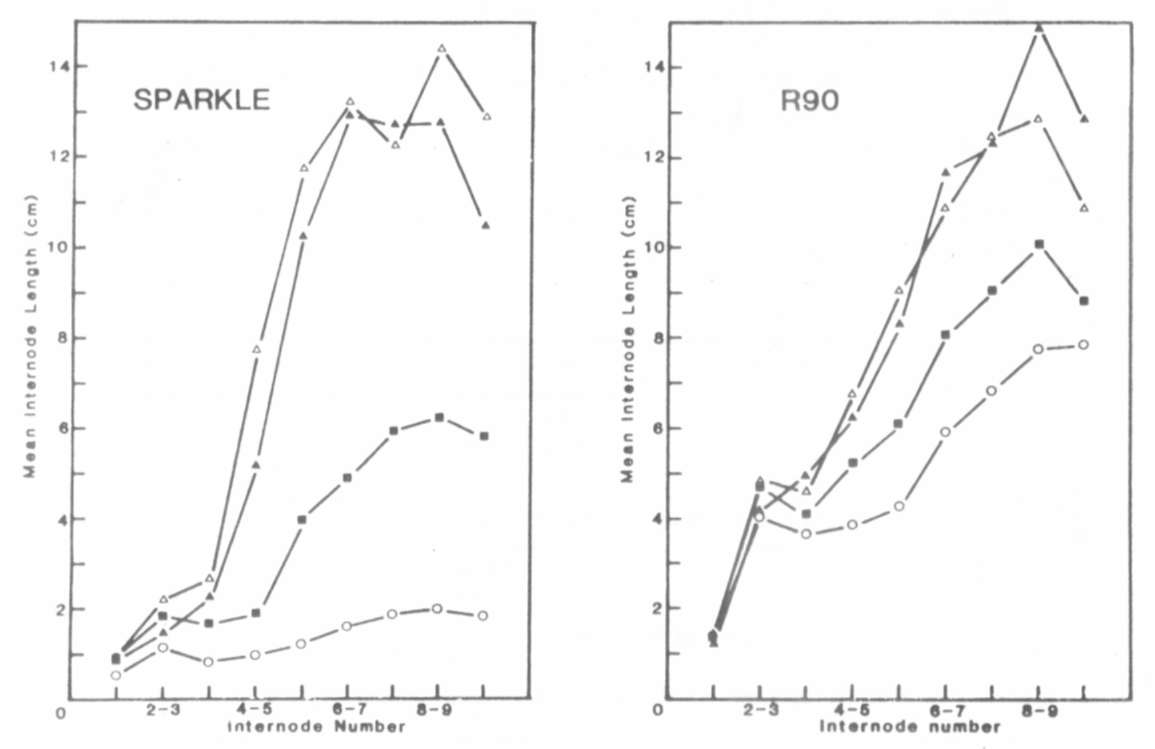
In conclusion, the increased
internode length of mutant R90
appears to be caused by a mutation at the la locus (la(R90))
which has a similar phenotypic effect to the previously described
la allele.
appears to be caused by a mutation at the la locus (la(R90))
which has a similar phenotypic effect to the previously described
la allele.
1. De
Haan, H. 1927. Genetica 9:481-497.
2. Ingram, T. J. and J. B. Reid. 1987. J. Plant
Growth
Regulation (in press).
Regulation (in press).
3. Ingram, T. J. and J. B. Reid. 1987. Plant
Physiol. 83:
in press).
in press).
4. Ingram, T. J., J. B. Reid, W. C. Potts, and I. C.
Murfet.
1983. Physiol. Plant. 59:607-616.
1983. Physiol. Plant. 59:607-616.
5. Ingram, T. J., J. B. Reid, I. C. Murfet, P.
Gaskin,
C. L. Willis, and J. MacMillan.
1984. Planta 160:455-463.
6. Jolly, C. J., J. B. Reid and J. J. Ross. 1987.
Physiol.
Plant. 69:489-498.
Plant. 69:489-498.
7. Potts, W. C, J. B. Reid and I. C. Murfet. 1985.
Physiol.
Plant. 63:357-364.
Plant. 63:357-364.
8. Rasmusson, J. 1927. Hereditas
10:1-150.
9. Reid, J. B. 1986. In A Genetic Approach to
Plant
Biochemistry, pp. 1-34. Eds. A. D. Blonstein and P. J. King,
Springer-Verlag, Wien.
Biochemistry, pp. 1-34. Eds. A. D. Blonstein and P. J. King,
Springer-Verlag, Wien.
10. Reid, J. B. and W. C. Potts. 1986. Physiol.
Plant.
66:417-426.
66:417-426.
11. Reid, J.
B., I. C. Murfet and W. C. Potts. 1983. J. Exp.
Bot. 34:349-364.
Bot. 34:349-364.
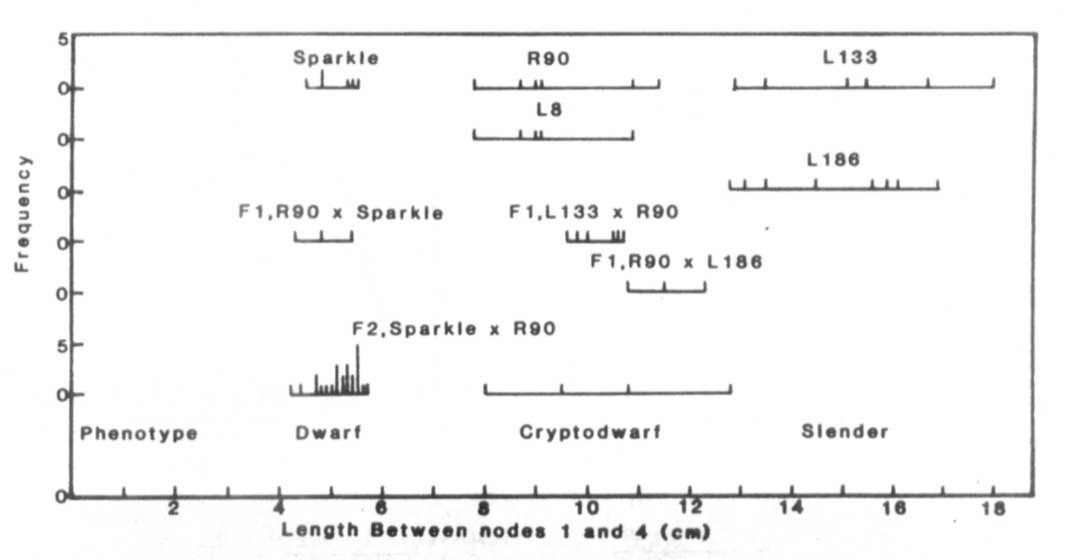
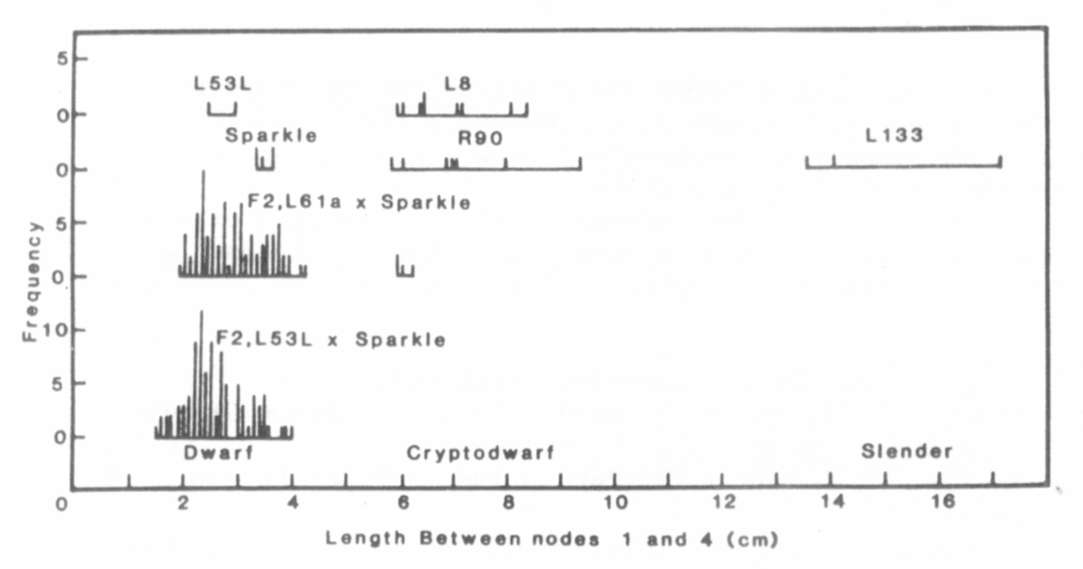
Fig. 1. The distribution of stem length
between nodes 1
and 4 for lines Sparkle (le La cryc), R90 (le la cryc),
L133 (le la crys), L8 (le la cryc) and L186 (Le la crys),
the F1 and F2 of cross Sparkle x R90 and the F1 of crosses
R90 x L133 and R90 x L186. The plants were grown under an 18 h photoperiod.
and 4 for lines Sparkle (le La cryc), R90 (le la cryc),
L133 (le la crys), L8 (le la cryc) and L186 (Le la crys),
the F1 and F2 of cross Sparkle x R90 and the F1 of crosses
R90 x L133 and R90 x L186. The plants were grown under an 18 h photoperiod.