CHARACTERS OF THE BIFURCATED MUTANT 157 OF GOTTSCHALK'S PISUM COLLECTION
G. WoLff Institute of Genetics, University of Bonn
Federal Republic of Germany
Mutant 157 exhibits stem bifurcation but because it is incompletely
penetrant the yield of this genotype varies in relation to the number of
bifurcated plants in the population (bifurcated plants produce more seed
than monopodial plants). Mutant 157 is reported to be a single-gene
mutant, designated bif-2 (1).
This genotype was analyzed for seed protein content after being
grown under relatively similar conditions in 1975 and 1976. We could
show that in both years the seed protein content of the mutant was sta-
tistically significantly higher than that of the initial line (DGV):
1975 - 113% of the initial line; 1976 - 111% (2). The same tendency was
evident in subsequent years though significant differences were not
found. A possible explanation for this was that the growing conditions
were not precisely comparable.
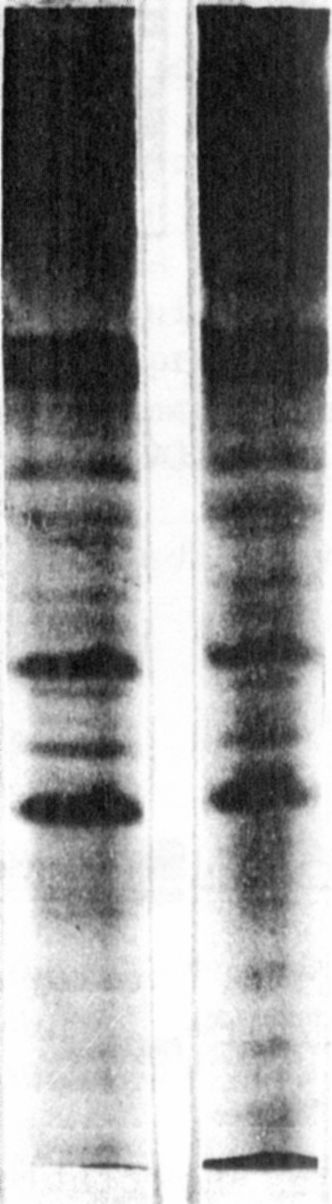
Investigations on the composition of the seed proteins showed that
the increased protein content of the mutant was mainly due to a 16% in-
crease in the albumin fraction over that of the initial line. The elec-
trophoretic separation of the albumins revealed a similar banding pat-
tern for both genotypes. Since no quantitative deviations in the sub-
fractions could be identified, it was proposed that in the mutant the
albumin fraction as a whole was increased (Fig. 1).
The enzymes catalase, alcohol dehydrogenase, ribonuclease,
fructose-1,6-biphosphatase, amylase, esterase, and certain glycoproteins
exhibited Identical phenotypes in both parental and mutant lines when
extracts were subjected to electrophoresis.
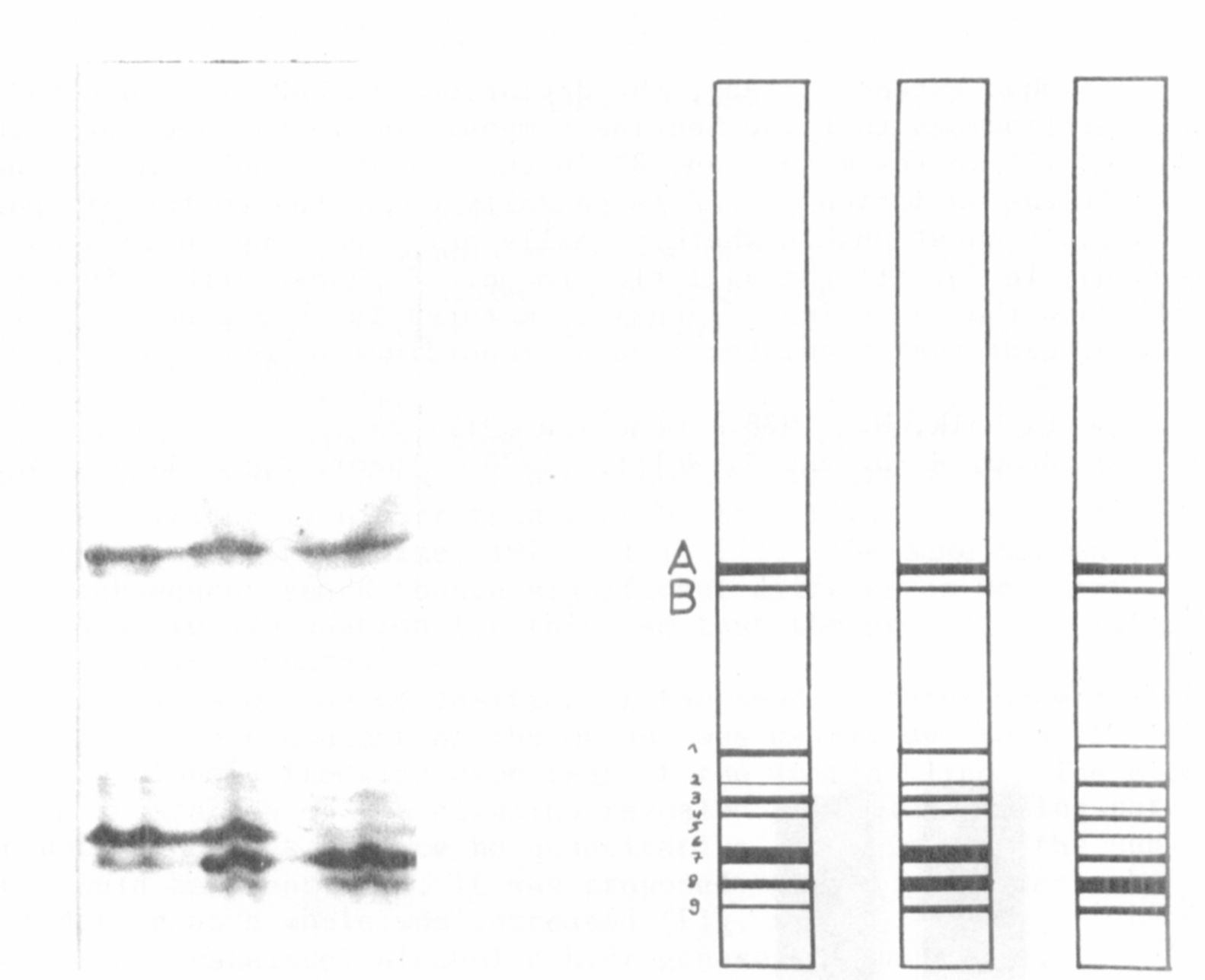
Pisum seeds also contain the enzyme leucine-amino-peptidase (LAP).
The pattern on the gel shows two bands (A and B) in the region of Rf-
values of about 0.55, a broad one and a faint one below. Both bands are
unchanged in the mutant. In the lower part of the gel (Rf-values
between 0.75 and 0.95) is a group of nine bands. Some differences
between the genotypes are evident (Fig. 3a, b). Band 7 of the initial
line is very prominent, whereas in the mutant it is reduced, and instead
hand 8 is prominent. There may be other differences as well but the
interpretation of these may not be reliable given the lack of clarity of
the gel.
We have crossed both of these genotypes. The middle column of
Fig. 1 a and b shows the pattern of the heterozygote genotype. The
upper bands (A and B) are identical to those of the parents while the
lowet part seems to show a heterozygous pattern. Bands 1 to 4 corres-
pond to those of the control genotype.
Possibly, gene bif-2 regulates the amount of a special subtraction
or component of the LAP in the mutant, reducing the quantity of the sub-
stance in position 7 while that in position 8 is increased. This
character is co-dominantly inherited in the F«j both alleles are ex-
pressed. In fractions 1 to 6, on the other hand, the dominant, influence
of the initial line is expressed.
To what extent, if any, the deviations in LAP are connected with
earlier findings that the leucine composition in the seed proteins is
altered (7% in the mutant and 8% in the initial line) remains unknown.
If the variation in LAP is causally connected to the bif phenotype
then it is questionable whether really only one gene in this mutant is
responsible for all the variation found. Perhaps further investigations
will show that a series of genes is mutated in this genotype, or that a