SOLUBLE AUXIN BINDING PROTEIN IN PEA?
Hajek, K. Institute of Genetics
University of Bonn, Federal Republic of Germany
As Dollstadt (1) already proved eight years ago, there are mem-
brane bound auxin binding proteins in pea. In 1981 Jacobsen (2)
showed the existence of auxin binding proteins in the cytoplasm of pea
epicotyls. The binding assay he used was the ammonium sulphate (AS)
precipitation. To confirm and to compare this method, some other
methods had been used and optimized. The soluble auxin binding pro-
teins (sABP) in the crude cytosol could be proved by three methods.
The PEI (polyethyleneimine)-treated filter test, used first by Bruns,
et al. (3) for estradiol receptors; a method using NC (Nitrocellu-
lose)-filters, which bind specifically proteins; and the AS-precipita-
tion method, all showed the existence of the sABP.
All calculations were carried out according to Scatchard (4).
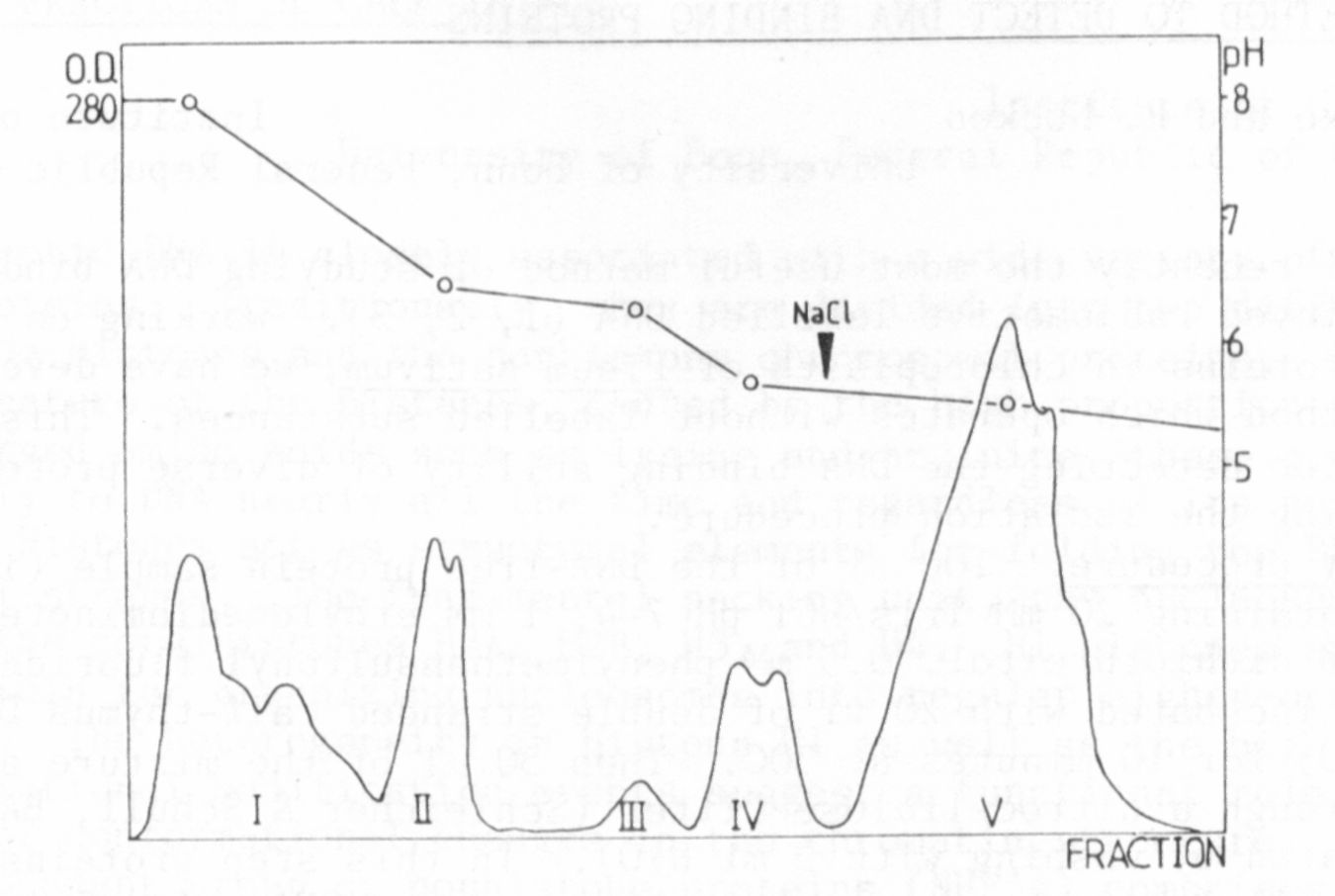
To get the sABP more purified, chromatofocusing (CF) was used as
a separation method for proteins (5). The crude cytosol was intro-
duced into a column filled with a CG-gel and the different proteins
were eluted according to their pI, and characterized by their respec-
tive elution pH. The elution pattern which was obtained is shown in
Fig. 1. The pattern was reproducible. This fact and the composition
of the eluted protein peaks was checked by SDS-electrophoresis.
Most of the binding appeared in peak IV, which is a double peak
with a pH range betwen 5.4 and 6.1. In this protein fraction sABP
could be proved by the AS-precipitation method, the PEI-treated filter
test, and NC-filter test, and also with the equilibrium dialysis.
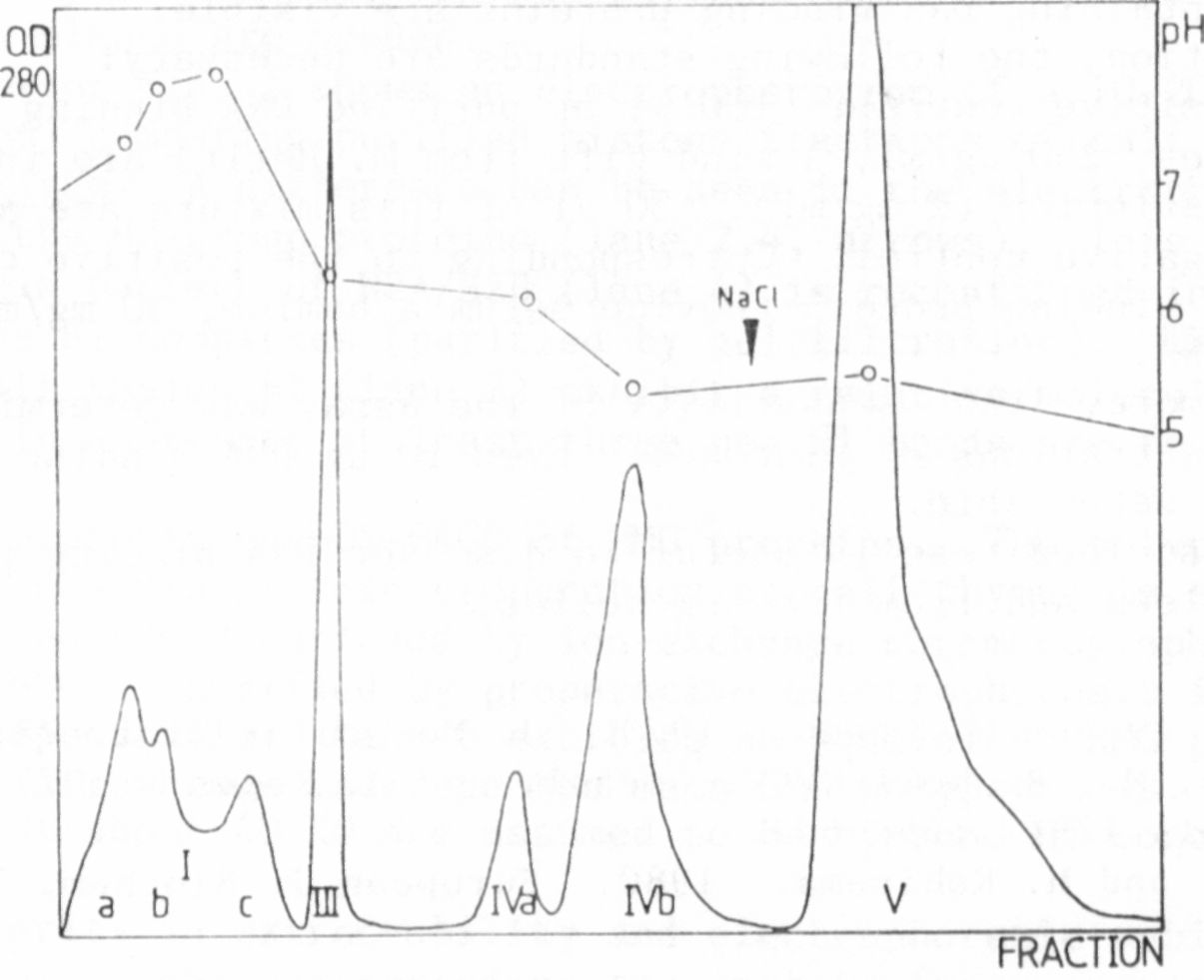
One complete run required up to four days, so it was obvious that
more satisfactory results would be obtained if first the duration of a
run could be shortened and second the resolution improved. This was
achieved using an HPLC-pump and a pressure resistant column. The
duration of one run could be shortened to one day and the double peak
IV was separated in two close but distinct peaks: peak IVa with pH 6.1
and peak IVb with pH 5.4 (Fig. 2).
The protein of the other peaks did not show any binding, but sABP
could be detected in the protein elution of peak IVa and peak IVb.
This could be shown by the AS-precipitation method, the PEI-treated
filter test, and the NC-filter test.
So there are at least two sorts of sABP in pea, which seem to be
time-dependent (6).
We intend to purify these most sensitive sABP in order to show
the influence on the gene activity in the cells of epicotyls from pea.
1. Dollstadt, R., K. Hirschberg, E. Winkler, and G. Hubnei. 1976.
Planta 130:105-111.
2. Jacobsen, H.-J. 1981. Cell Biol. Intern. Rep. 5:768.
3. Bruns, R., K. Lawson-Wendling, and T. Pugsley. 1983. Anal.
Biochem. 132:74-81.
4. Scatchard, G. 1949. Ann. N.Y. Acad. Sci. 51:660-672.
5. Sluyterman, L. A. 1.982. TIBS, May 1982
6. Jacobsen, H.-J. 1984. Plant and Cell Physiol. 25:867-873.