PNL Volume 16
1984
RESEARCH REPORTS
25
SELECTION OF NODULATION RESISTANT MUTANTS OF PEA
Jacobsen, E.
Dept. of Genetics, University of Groningen
Haren, The Netherlands
Legumes are capable of fixing with nodules containing bacteriods
of Rhizobium, a symbiosis which is affected by several genes of both
partners. Moreover, natural variants and mutants that negatively affect
nodulation and/or N2 fixation have been found in both partners. In pea,
natural variation with respect to nodulation has been described (1,2)
and recently nodulation resistance has been found in material after
mutagenic treatment (3). In the present contribution, the successful
selection of other nodulation resistant mutants is reported. Selection
of these mutants was carried out in M2 families which had been used
before for the isolation of nitrate reductase deficient mutants (4) and
a mutant, nod-3, highly nodulating in the presence of nitrate (5).
Seeds were germinated in moistened vermiculite, and after one week,
seedlings were transferred to aerated nitrogen-free liquid medium inocu-
lated with Rhizobium leguminosarurn strain PF2. Sixty seedlings were
grown per plastic container, including 10 plants of the parent variety
'Rondo'. The latter were included to verify the effectiveness of the
infection. Eighteen days after sowing, Rondo seedlings were clearly
nodulated. Twenty-eight days after sowing, M2 seedlings were screened
for nodulation. Plants with well-developed roots but without nodules,
from containers in which all 10 control plants had nodulated well, were
transferred to aerated nitrogen containing liquid medium for seed
multiplication. In all, 20 plants were selected from among 250
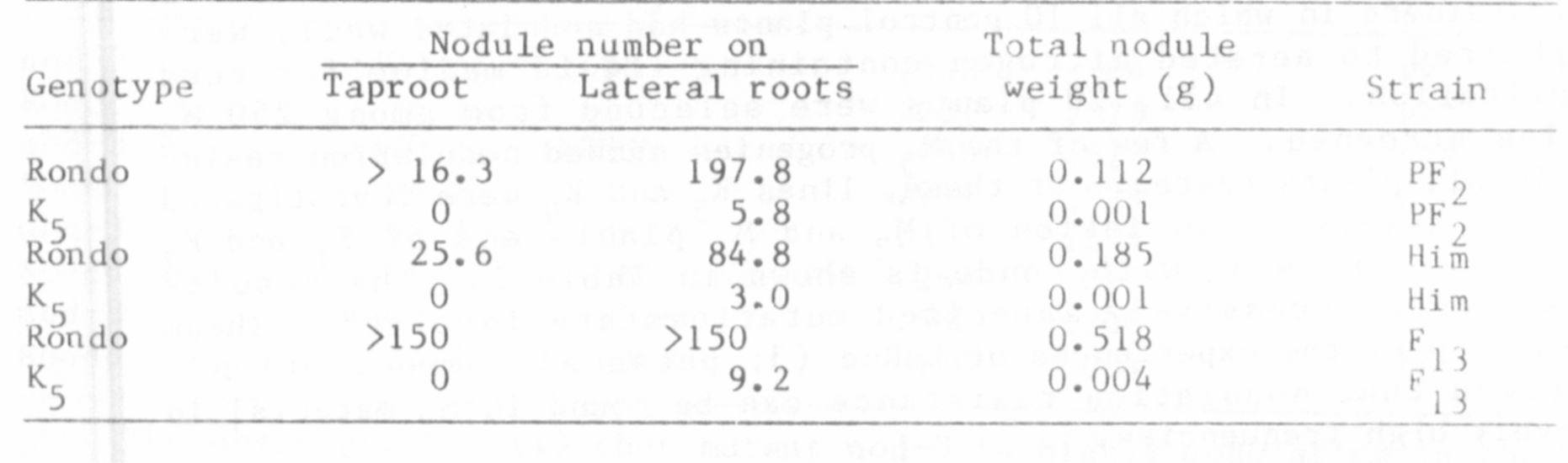
families screened. A few of the M3 progenies showed nodulation resis-
tance in all plants tested. Of these, lines and were investigated
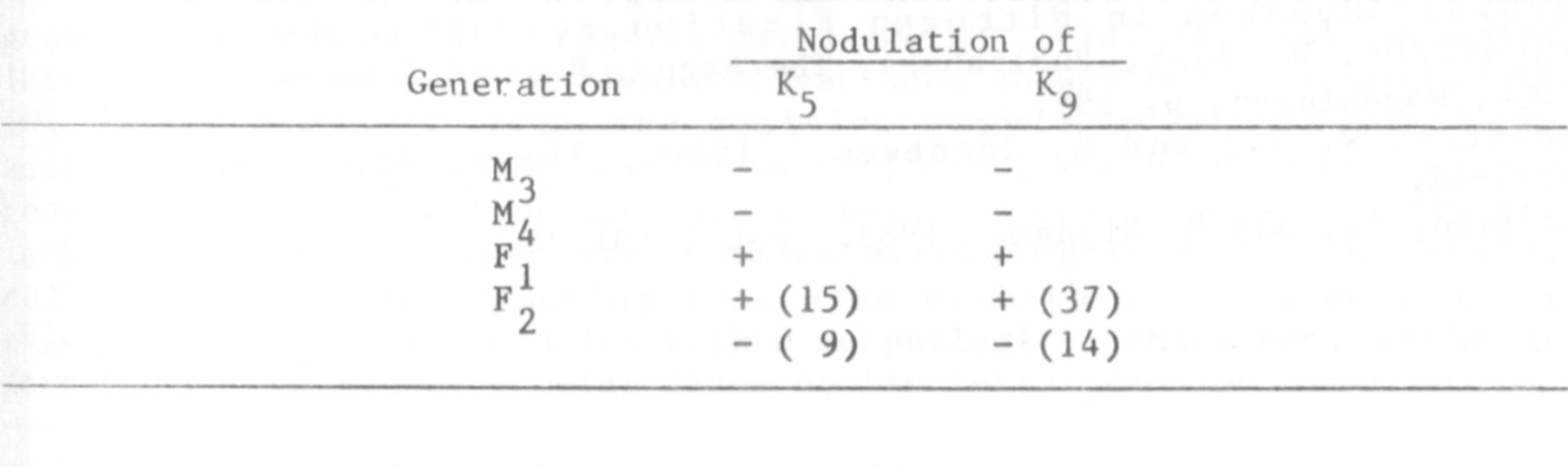
more extensively. Nodulation of M3 and M4 plants and of F1 and F2
plants after crossing with Rondo is shown in Table 1. The results
indicate that recessively inherited mutations are involved. These
results support the experiences of LaRue (3; personal communication),
who showed that nodulation resistance can be found in M2 material in
relatively high frequencies.
Table 1. Nodulation of nodulation resistant lines K5 and K9 in the M3
and M4 generation, and in the F1 and F2 after crossing with Rondo after
inoculation with R. leguminosarum strain PF2.