POLYPEPTIDE COMPOSITION OF PROTEIN FRACTIONS FROM PISUM SEEDS AND PODS
DURING DEVELOPMENT
Gaul, E. Institute of Genetics, University of Bonn
Federal Republic of Germany
Quantitative differences (relative and absolute) in protein in the
pods and seeds of 'Dippes Gelbe Viktoria' were shown to vary with
developmental age (1). Qualitative changes in pod and seed protein
during development were also investigated, using Na-dodecyl sulfate
polyacrylamide gel electrophoresis (SDS-PAGE). Albumins and globulins,
extracted from seeds, and the water soluble and water insoluble proteins
from pods were fractionated and analyzed.
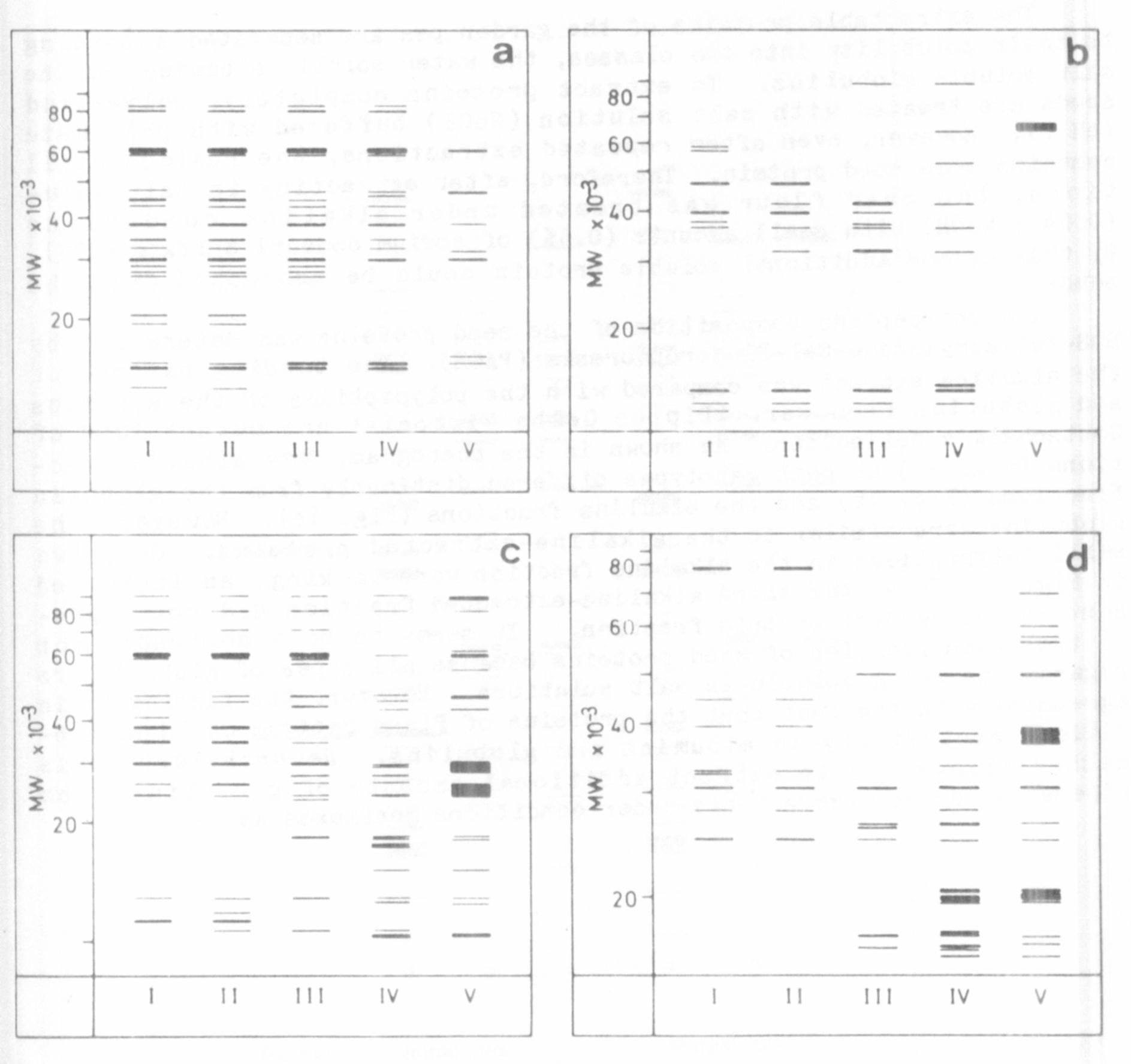
The water soluble proteins of the pods represent a heterogeneous
fraction (Fig. 1a). However, the polypeptide patterns exhibited only
small variation during development. In all stages investigated, a
polypeptide with a molecular weight of 60 KD dominated. In the dry pods
the number of bands was remarkably reduced.
A less complex protein pattern was characteristic for the water in-
soluble proteins (Fig. 1b). In the early developmental stages,
polypeptides with molecular weights in the range of 12-14 and 35-48 KD
were predominant. Only high molecular subfractions were detected in
senescent pods.
The seed albumins in the early stages of development highly coin-
cided with the water soluble fraction from pod proteins (Fig. 1a, c).
In later stages the special demands on the metabolic processes of seed
development were expressed by a drastic change of polypeptide
composition. This was especially conspicuous in the case of the typical
albumin bands (25 and 30 KD). The polypeptides were predominant during
maturation, whereas they were only slightly stained in the earlier
stages (Fig. 1c). The accumulation of storage proteins was likewise ac-
companied by alterations during successive stages of development (Fig.
1d). In the beginning, high molecular weight bands as well as polypep-
tides in the range of 25-40 KD were detected. The vicilin components of
32 and 50 KD could be recognized prior to the legumin subunits with
molecular weights of 20 and 40 KD. Several additional bands were found,
especially in the low molecular weight range. In dry seeds a polypeptide
of 71 KD appeared which was recently identified as a subunit of con-
vicilin by Croy et al (2).