PNL Volume 13 1981
RESEARCH REPORTS
47
|
|
||
|
46 RESEARCH REPORTS PNL Volume 13 1981
|
||
|
|
||
|
GENOTYPIC VARIATION IN THE FLOWER DELAYING EFFECT OF ETHEPHON
Reid, J. B. Botany Department, University of Tasmania, Hobart, Australia
The ethylene releasing compound ethephon is a potent inhibitor of flower
initiation in the early developing line of peas, line 58 (6). However, an
endogenous role for ethylene in the control of flowering in peas has not yet
been found (7). For this reason it was decided to examine how a range of
flowering genotypes responded to applied ethephon, with the hope that some
correlation between the magnitude of the flowering response and the flowering
genotype could be found.
The growing techniques used were similar to those previously used at
Hobart (2, 5). Treatment with ethephon was performed by applying 10 mkl of
ethanol containing the required quantity of ethephon to the dry testa. After
the ethanol evaporated the seeds were planted 2 cm beneath the surface of
the growth medium. Plants decotyledonized after 18 h imbibition were grown
on White's nutrient agar medium until leaf 4 was fully expanded.
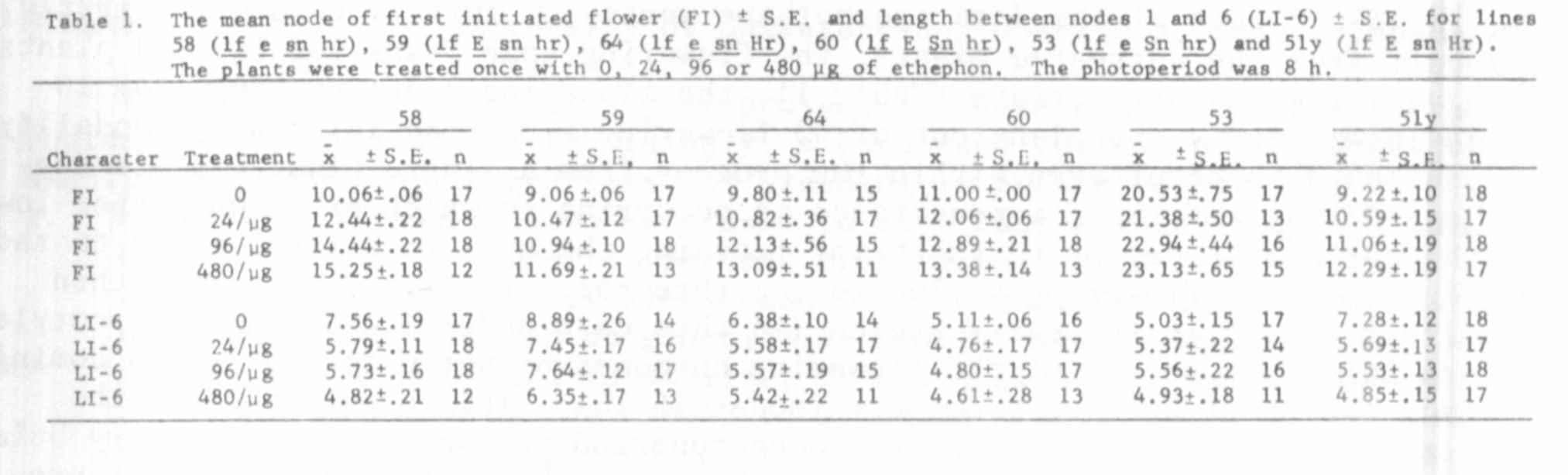
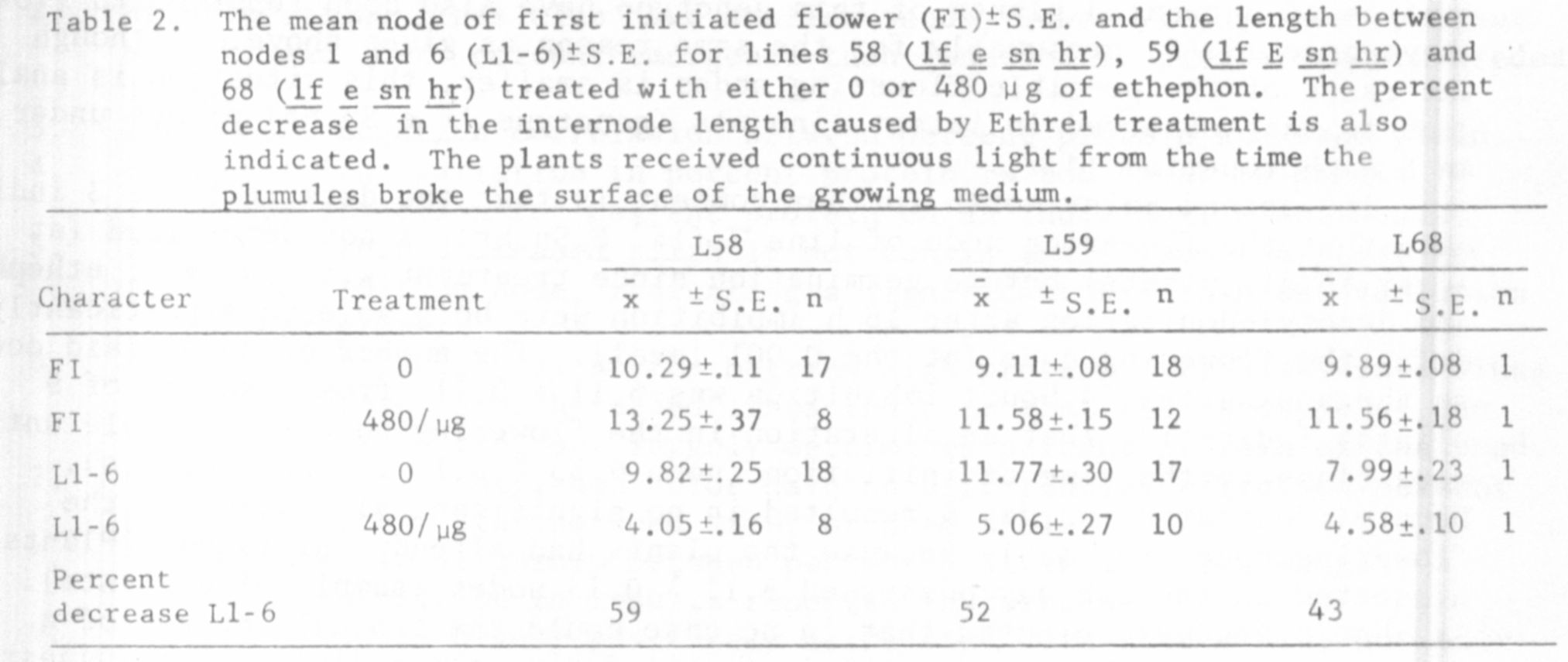
The results in Tables 1, 2, and 3 show that ethephon is capable of in-
creasing the flowering node of lines 58 (flowering genotype lf e sn hr),
59 (lf E sn hr), 64 (lf E sn hr), 60 (lf E Sn hr), 53 (lf e Sn hr),
51 (lf E sn Hr), and 7 (lfa E Sn hr) under an 8 h photoperiod and of lines
58, 68 (lf e sn hr), and 59 under continuous light (all delays significant
at the 0.001 level when 480 ug of ethephon was used). However, although
ethephon is general in its ability to delay the flowering node, the size of
the delay varied considerably from one line to another within one experiment.
For example, the three phenotypically similar lines, 58 (lf e sn hr),
68 (lf e sn hr), and 59 (lf E sn hr) differed significantly in the extent
they were delayed by ethephon (Tables 1 and 2). These differences in the
flowering response between the lines did not appear to be directly associated
with the presence or absence of the individual genes E, Sn, or Hr. Further,
the balance of the flowering hormones existing in the plant during the early
growth did not appear to be implicated in the differential response in the
early region since line 58 plants were delayed to a later node than were
line 60 plants even though line 60 cotyledons and shoots have been shown to
produce a more inhibitory balance of the flowering hormones than line 58 (2,
8). It appears other as yet undetermined genetic systems are responsible
for the largest part of these different responses as illustrated by the degree
of difference between lines 68 and 58 (both genotype lf e sn hr). Consequently,
the present study does not indicate where, if at all, endogenous ethylene
plays a role in controlling flowering in peas.
As well as having varying effects on the size of the flowering delay
it is interesting to note that a particular concentration of ethephon also
had differing effects on the vegetative growth of the different lines. This
is illustrated by the fact that the length between nodes 1 and 6 was consis-
tently reduced by the greatest percentage in line 58 (Tables 1 and 2). How-
ever, this measurement does not appear to tell the whole story, since plants
of lines 58 and 55 were very "sick" in appearance when treated with 480 ug
of ethephon even after 4 or 5 weeks growth, while lines 51y and 68 appeared
almost unaffected by this treatment at this time. Lines 60, 59, and 64 were
somewhat intermediate in their response between these two groups. Whether
this differing vegetative response to ethephon is responsible for the differing
flowering responses (e.g. between lines 58 and 68) is unclear.
|
||
|
|
||
|
|
||||
|
PNL Volume 13 1981
|
RESEARCH REPORTS
|
47
|
||
|
|
||||
 |
||||
|
|
||||
 |
||||
|
|
||||
 |
||||
|
|
||||
|
|
||||
|
43 RESEARCH REPORTS
|
PNL Volume 13
|
1981
|
||
|
|
||||
|
Two facets of the data are perhaps worthy of further comment. Firstly,
apart from the untreated plants, the flowering node means for line 64 plants
have large standard errors (Table 1), the flowering nodes varying from 10
to 16 with only one plant out of 43 flowering from node 14. This bimodality
continued to occur even within the progeny from a single plant. It is sug-
gested that a form of impenetrance is occurring in which ethephon either lowers
the ratio of promotor to inhibitor reaching the apex to a level close to the
threshold for flowering or lowers the threshold itself. Plants will then
either flower in the early region (in this case nodes 10-13) when the cotyledon
are the major source of the flowering hormones or not until the ratio coming
from the shoot becomes promotory (nodes 15-18). This occurs because the
cotyledons of line 64 under an 8 h photoperiod produce a more promotory balance
of the flowering hormones than does the young shoot (5). A very small pro-
portion of untreated plants of this genotype have also been reported to flower
above node 15 (3) presumably for the same reason as given above. Although
the range of the possible flowering nodes is smaller, this situation is analo-
gous to that observed in intact line 61a (genotype lf e Sn hr) plants under
an 8 h photoperiod (4,7).
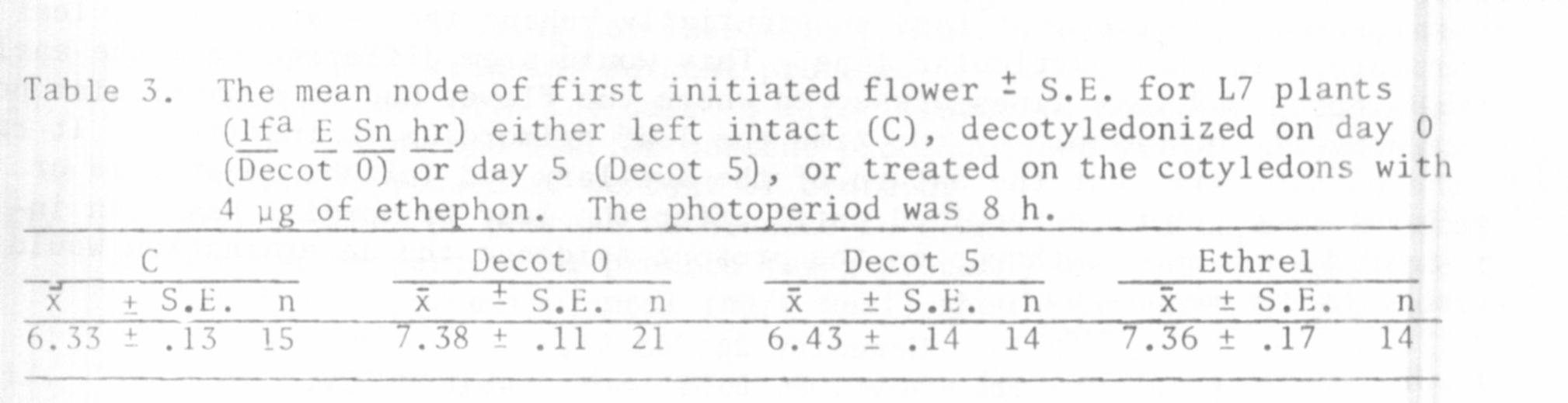
The second point worth further comment is that the data in Table 3 indi-
cate that the flowering node of line 7 (lfa E Sn hr) is not determined (at
least in all plants) before germination since treatment with 80 mkg of ethephon
and decotyledonization after 18 h imbibition were both able to significantly
delay the flowering node (at the 0.001 level). The number of nodes laid down
in the apex after 24 hours imbibition was 6.11 +/- 0.11 (from a sample of 9
plants) indicating that an alteration in the flowering node is possible until
very close to the time of initiation (node 6.33 +/- 0.13 in the controls).
Decotyledonization on day 5 resulted in no significant alteration of the
flowering node presumably because the plants had already initiated. Plants
dissected on the 5th day possessed 8.13 +/- 0.13 nodes (sample of 8 plants).
It should however be noted that in no case could the typical "bulge" of a
flower primordium be seen in the leaf axil during these dissections suggesting
axillary bud development lags substantially behind the development of leaf
primordia in this particular line. This would seem different from the early
developing and late lines dissected where the flower bud at a particular node
is normally observable by the time the leaf primordium is initiated. It raises
the possibility that the nature of the axillary bud (either vegetative or
floral) may not be determined until after the leaf primordium has been in-
itiated in line 7 although on the present evidence the determination would
be made before day 5.
1. Murfet, I. C. T971a. Heredity 26:243-57.
2. Murfet, I. C. 1971b, Aust. J. Biol. Sci. 24:1089-101.
3. Murfet, I. C. 1973a. Heredity 31:157-64.
4. Murfet, I. C. 1973b. Aust. J. Biol. Sci. 26:669-73.
5. Reid, J. B., 1979. Ann. Bot. 44:163-73.
6. Reid, J. B. and 1. C. Murfet. 1974. Aust. J. PI. Physiol. 1:591-4.
7. Reid, J. B. and I. C. Murfet. 1980. Ann. Bot. 45:583-6.
Wall B., J. B. Reidv and I. C. Murfet. 1974. PNL 6:50-51.
|
||||
|
|
||||