58 RESEARCH REPORTS
PNL Volume 12
1980
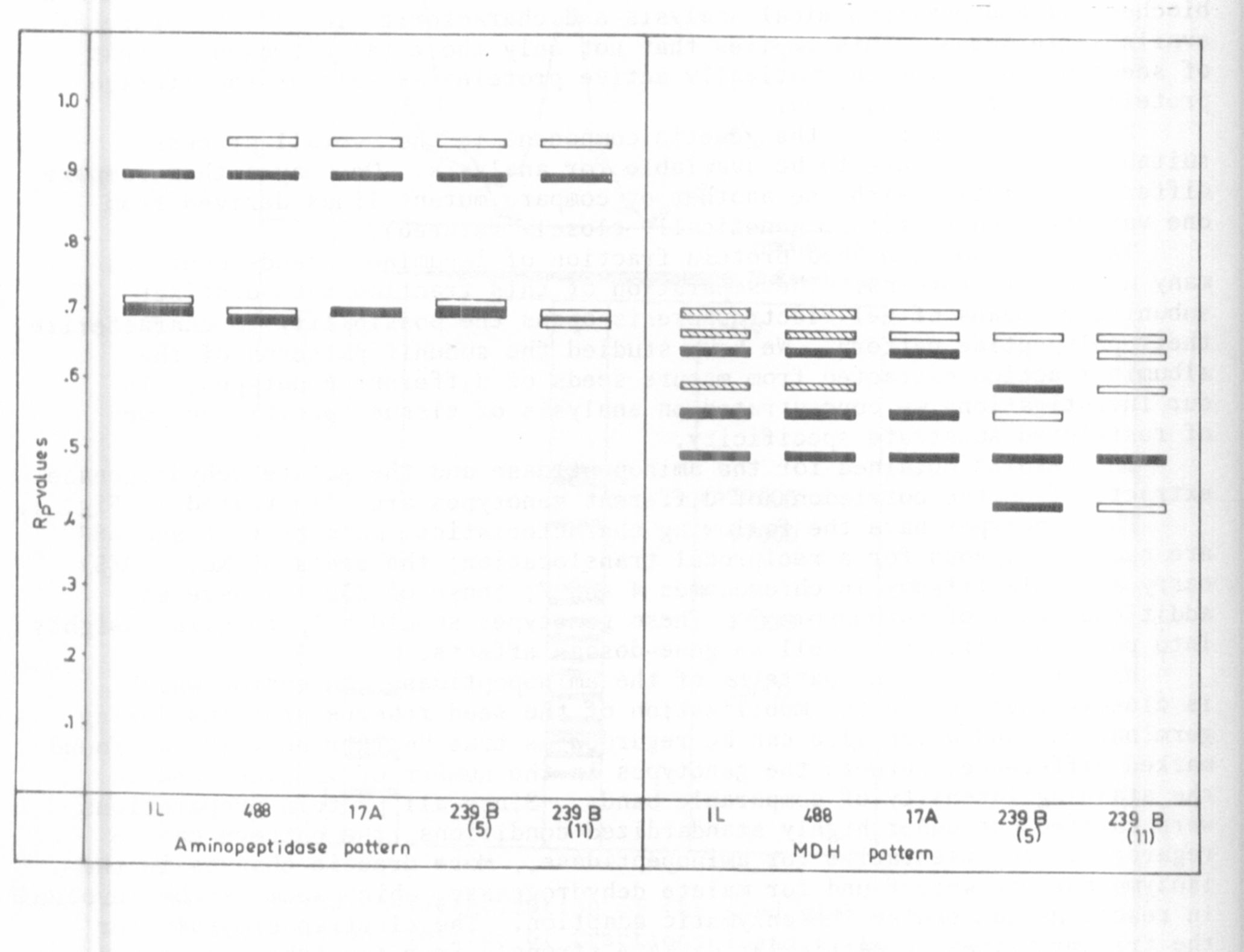
Tg. 1. Aminopeptidase and malate dehydrogenase banding patterns in seeds
of different pea genotypes.
|
|
||
|
PNL Volume 12 1980 RESEARCH REPORTS 57
|
||
|
|
||
|
CHARACTERIZATION OF TISSUE-SPECIFIC SEED PROTEINS IN PISUM
Muller, H.P. Institute of Genetics, University of Bonn, West Germany
A realistic estimation of an expected increase in protein quantity and
quality of crop plants depends on an understanding of the genetic control
of protein structures as well as of the regulatory processes during the
incorporation of functional units involved in the synthesis, processing, and
deposition of seed proteins.
A study for elucidating some of the problems indicated involves detailed
biochemical and physiochemical analysis and characterization of the proteins
available in seeds. This implies that not only the total nitrogen content
of seeds but also the enzymatically active proteins as well as the storage
proteins have to be analyzed.
For an assessment of the genetic component in the overall process,
suitable genotypes have to be available for analysis. One may either compare
different varieties with one another or compare mutant lines derived from
one variety (and therefore genetically closely related).
The water soluble seed protein fraction of leguminous seeds contains
many different proteins. The separation of this fraction into distinct
subunits by means of gel-electrophoresis opens the possibility to characterize
their polypeptide pattern. We have studied the subunit patterns of the
albumin fraction extracted from mature seeds of different genotypes. In
our investigations we concentrated on analysis of tissue-specific enzymes
of restricted substrate specificity.
The results obtained for the aminopeptidase and the malate dehydrogenase
extracted from the cotyledons of different genotypes are illustrated in Fig. 1.
The genotypes have the following characteristics: mutants 17 A and 488
are each homozygous for a reciprocal translocation; the seeds of No. 239(5)
carry a double trisomy in chromosomes 4 and 7; those of 239(11) have an
additional copy of chromosome 7. These genotypes should help to gain insights
into position effects as well as gene-dosage effects.
With respect to the patterns of the aminopeptidase, an enzyme which
is closely involved in the mobilization of the seed reserve proteins during
germination, and which also can be regarded as true "marker enzyme", we found
marked differences between the genotypes in the number of isozymes and in
the staining intensity of comparable bands. Since all protein preparations
were carried out under highly standardized conditions, the pattern can be
regarded as representative for aminopeptidase. More drastic changes in the
isozyme pattern were found for malate dehydrogenase, which seems to be involved
in reactions concerning the enzymatic adaption. The electropherograms for
the trisoinic lines in particular deviate strongly from the other genotypes.
There are some common bands, but others are lacking or are additionally
detectable. The results show that considerable heterogeneity of aminopeptidase
and MDH-isozymes, as judged by variance in the polypeptide patterns, is
produced by or associated with the genetic differences. There are some
indications of structural modifications of enzymes. Polymorphic forms of
enzymes seem to have adaptive significance. Furthermore, the knowledge of
the presence of enzymatically active proteins found in seeds should constitute
a useful basis for studying the metabolic events during seed germination.
|
||
|
|
||
|
|
||||
|
58 RESEARCH REPORTS
|
PNL Volume 12
|
1980
|
||
|
|
||||
 |
||||
|
|
||||
|
Tg. 1. Aminopeptidase and malate dehydrogenase banding patterns in seeds
of different pea genotypes.
|
||||
|
|
||||