ISOLATION OF THE STORAGE PROTEINS OF PEA SEEDS
MUller, H. P. Institute of Genetics, University of Bonn, West Germany
Legume cotyledons accumulate during their development both albumins
and globulins. The latter constitute a protein reserve deposition which
serves the nutritional requirements of the developing embryo during germination.
The incorporation of proteins into the cotyledon is governed by a genetically
regulated protein-synthesizing system, the mechanism of which is unknown.
The globulins are proteins with particular physical properties exhibiting
heterogeneity with regard to their subunit composition. Since most of the
cotyledon proteins are coded for by nuclear DNA of the embryo, the storage
proteins constitute an especially valuable system for analyzing the control
mechanism of the genome as well as the physiological influence of the seed-
bearing plant.
For understanding the controlling mechanism responsible for the synthesis
of specific proteins in the cotyledons we first must have an idea of the
different protein species found within the seeds. Then it is important to
isolate, purify, and finally to biochemically characterize those proteins
in detail.
Using SDS-gel-electrophoresis for analyzing the purified globulin frac-
tion of pea seeds, one obtains a genotype-specific polypeptide pattern.
Analyzing seeds of mutants of the same variety presents considerable
difficulties witli regard to quantitative extraction of proteins. The:extrac-
tion methods commonly used for Phaseolus and Vicia are not well suited for
extracting Pisum seed proteins. Therefore, it was necessary to develop an
extraction method especially adapted for pea seed proteins: the proteins
were first extracted at the isoelectric point (IEP) at low salt concentration
(acid extraction), then under alkaline conditions. This procedure allowed
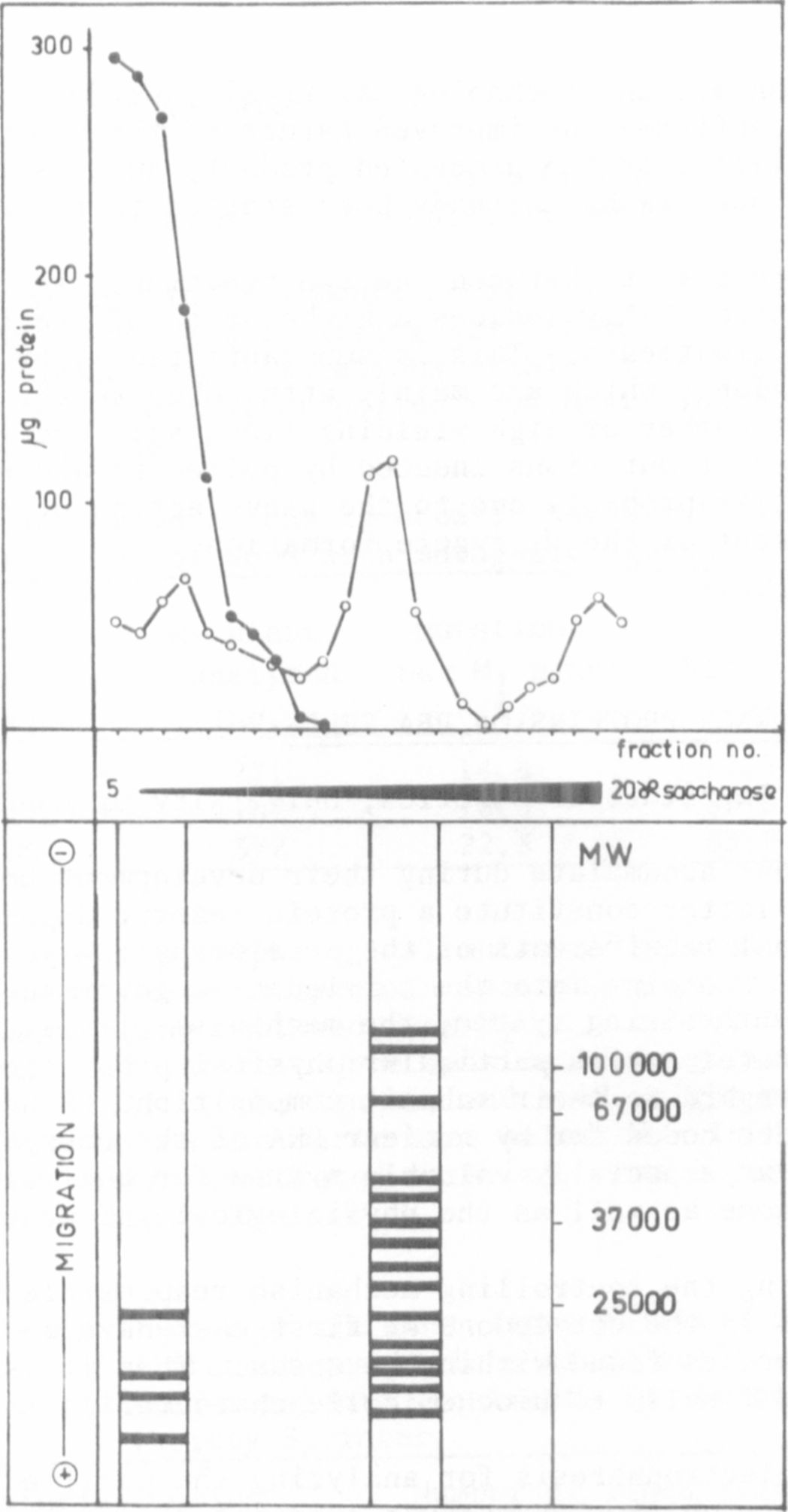
100% recovery. Sucrose gradient analysis of the two extracts resulted in
two dilution profiles as demonstrated in Fig. 1.