NEW TECHNIQUES TO SCREEN PEA SEEDLINGS FOR RESISTANCE TO SCLEROTINIA WHITE MOLD
Blanchette, B. L. and D. L. Auld University of Idaho, Moscow, ID U.S.A.
Sclerotinia white mold can be a devastating disease of peas, especially
under irrigated conditions. The causal fungus, Sclerotinia sclerotiorum,
produces hard black structures (sclerotia) which allow the pathogen to survive
in the soil for as long as 10 years. Whenever cool moist periods prevail,
the sclerotia germinate, giving rise to mushroom-like apothecia which form
billions of spores that are disseminated by wind. Pea plants are susceptible
to infection by these spores at all stages of growth. The initial symptoms
are water-soaked lesions which usually occur at the base of the stem. Under
warm humid conditions the lesions expand rapidly, which causes rotting of
the lower leaves and girdling of the stem. Wilting and the death of the plant
usually follow.
Field evaluation of pea genotypes for resistance to S. sclerotiorum
is difficult. The sporadic nature of the disease necessitates numerous
replications to detect significant genotypic differences. The plot size
in Sclerotinia white mold trials must also be great enough to test the effect
of canopy structure on disease avoidance since the microclimate under the
plant canopy can affect the incidence of white mold (2). Because of the
problems encountered in screening under field conditions, several screening
techniques were developed to allow preliminary evaluations for disease resis-
tance in the greenhouse and laboratory.
Breeding lines and plant introductions were screened in greenhouse mist
chambers maintained at approximately 18C and 90% relative humidity (1). Ten
days after planting, the pea seedlings were predisposed by misting for 24
hours. The predisposed plants were inoculated by placing one Sclerotinia-
infested oat kernel in contact with the base of each stem.
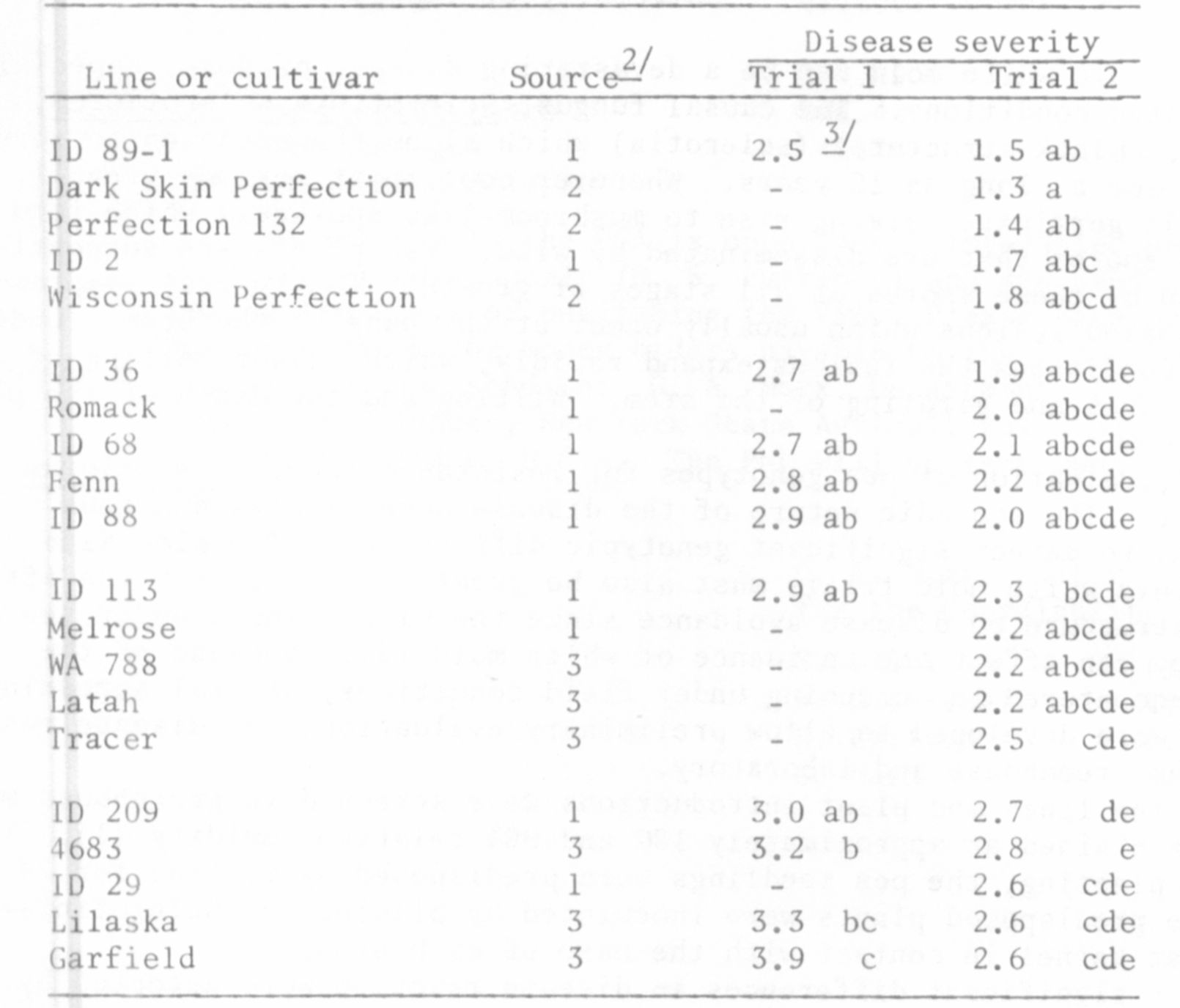
Highly significant differences in disease reaction were detected by
visually scoring the length of stem lesions three days after inoculation
(Table 1). The pea variety 'Garfield' was found to be very susceptible to
white mold and was used as the susceptible check in all greenhouse screenings.
Lines with lesions 50% shorter than Garfield were considered resistant. A
winter-hardy pea, ID 89-1 (which was selected from a 'Perfection' x AWP cross),
and several genotypes from a Perfection origin were found to be resistant.
The results of two separate screening trials were highly correlated (r= 0.84)
indicating this method was repeatable. Although most of the 388 plant intro-
ductions tested were susceptible to S. sclerotiorum in the greenhouse, several
lines exhibited a level of resistance equal to that found in Perfection (Table
A faster and simpler technique for testing plants for resistance to
S. sclerotiorum was developed using culture filtrates of the fungus. Flasks
containing a basal salt medium (3) with 1% pectin were inoculated with a
mycelial plug and incubated in a shake culture for six days at room temperature
The mycelium was filtered through cheesecloth and 20 ml of the filtrate placed
in vials. Six cm tall plants were removed from the vermiculite growing media,
washed, and the stem excised just above the seed while immersed in water.
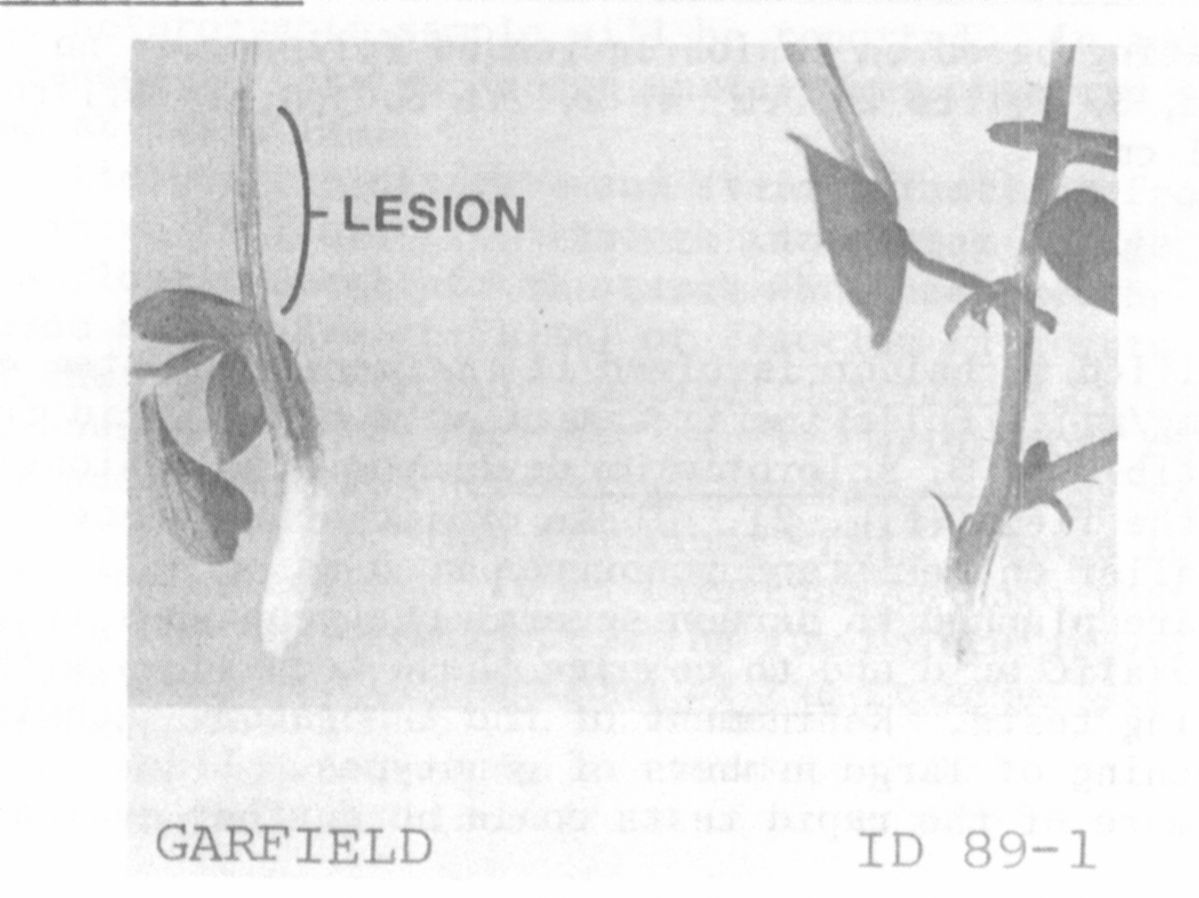
The stem was then placed in the fungus filtrate. The pea genotype ID 89-1
which had been resistant to the fungus in the greenhouse test did not wilt
within 4 hours, whereas the susceptible 'Lilaska' wilted severely in the
same time period (Fig. 1).